The Crystal Structure of Ramsdellite from Pirika Mine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JAN Iia 20U T6T1/V
IN REPLY REFER TO: UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY WASHINGTON 25. D.C. November 19, 1956 AEC-193/7 Mr. Robert D. Nininger Assistant Director for Exploration Division of Bav Materials U* S. Atomic Energy Commission Washington 25, D. C, Dear Bobs Transmitted herewith are three copies of TEI-622, "The crystal chemistry and mineralogy of vanadium," by Ho-ward T. Evans, Jr. Me are asking Mr. Hosted to approve our plan to publish this report as a chapter of a Geological Survey professional paper on miner alogy and geochemistry of the ores of the Colorado Plateau. Aelrnovledg- ment of AEC sponsorship will be made in the introductory chapter* Sincerely yours, r ^ O U—— TV , Z^*i—w«__ ™~ W. H. Bradley Chief Geoldigist .JAN iia 20U T6T1/V Geology and 8$i:aeralQgy This document consists of k-2 pages* Series A» Howard T. Erans, Jr, Trace Elements Investigations Report 622 This preliminary report is distributed without editorial and technical review for conformity with official standards and nomenclature. It is not for public inspection or quotation* *This report concerns work done on behalf of the Division of Raw Materials of the U. S« Atomic Energy Commission* - TEI-622 AHD MIHERALQ6T Distribution (Series A) Ro. of copies Atomic Energy Commission, Washington .»**«»..*»..«*»..««..*... 2 Division of Rs¥ Materials, Albuquerque ,...****.*.«.»*.....*.. 1 Division of Raw Materials, Austin »«,..«.»...»*.»...*«..*...«» 1 Diylsion of Raw Materials, Butte *«*,.»».*..,*...».»......*.*. 1 Division of Raw Materials, Casper ............a............... 1 Division of Raw Ifeterials, Denver ,........»...».....«.,.*..... 1 Division of Raw Materials, Ishpeming .................a....... 1 Division of Raw Materials, Pnoenix ...a.....,....*........*... 1 Division of Eaw Materials, Rapid City ....................... -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Investigations of Mixed-Anion Analogs of Manganite Perovskites and Bimetallic
Investigations of Mixed-Anion Analogs of Manganite Perovskites and Bimetallic Group II Nitride Fluorides By Oscar Kipruto Keino Submitted in Partial Fulfillment of the Requirements For the Degree of Master of Science in the Chemistry Program YOUNGSTOWN STATE UNIVERSITY December, 2017 Investigations of Mixed-Anion Analogs of Manganite Perovskites and Bimetallic Group II Nitride Fluorides By Oscar Kipruto Keino I hereby release this thesis to the public. I understand that this thesis will be made available from the Ohio LINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: ________________________________________________________________ Oscar Kipruto Keino, Student Date Approvals: ________________________________________________________________ Dr. Timothy R. Wagner, Thesis Advisor Date ________________________________________________________________ Dr. Sherri Lovelace-Cameron, Committee Member Date ________________________________________________________________ Dr. Allen Hunter, Committee Member Date ________________________________________________________________ Dr. Salvatore A. Sanders, Date Dean, College of Graduate Studies iii ABSTRACT Lanthanum manganites are perovskite related materials known in particular for their colossal magnetoresistance (CM) properties. Manganite compositions showing CM behavior are mixed cation compounds such as (CaxLa1-x) MnO3, which contain Mn ions in mixed -

Mineralogy and Metallogenesis of the Sanbao Mn–Ag (Zn-Pb) Deposit in the Laojunshan Ore District, SE Yunnan Province, China
minerals Article Mineralogy and Metallogenesis of the Sanbao Mn–Ag (Zn-Pb) Deposit in the Laojunshan Ore District, SE Yunnan Province, China Shengjiang Du 1,2, Hanjie Wen 3,4,*, Shirong Liu 3, Chaojian Qin 3, Yongfeng Yan 5, Guangshu Yang 5 and Pengyu Feng 5 1 State Key Laboratory of Nuclear Resources and Environment, East China University of Technology, Nanchang 330013, China; [email protected] 2 Chinese Academy of Geological Science, Beijing 100037, China 3 State Key Laboratory of Ore Deposit Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang 550081, China; [email protected] (S.L.); [email protected] (C.Q.) 4 University of Chinese Academy of Sciences, Beijing 100049, China 5 Kunming University of Science and Technology, Kunming 650093, China; [email protected] (Y.Y.); [email protected] (G.Y.); [email protected] (P.F.) * Correspondence: [email protected] Received: 26 June 2020; Accepted: 17 July 2020; Published: 23 July 2020 Abstract: The Sanbao Mn–Ag (Zn-Pb) deposit located in the Laojunshan ore district is one of the most important deposits that has produced most Ag and Mn metals in southeastern Yunnan Province, China. Few studies are available concerning the distribution and mineralization of Ag, restricting further resource exploration. In this study, detailed mineralogy, chronology, and geochemistry are examined with the aim of revealing Ag occurrence and its associated primary base-metal and supergene mineralization. Results show that manganite and romanèchite are the major Ag-bearing minerals. Cassiterite from the Mn–Ag ores yielded a U–Pb age of 436 17 Ma, consistent with ± the Caledonian age of the Nanwenhe granitic pluton. -

Manganese Deposits of Western Utah
Manganese Deposits of Western Utah GEOLOGICAL SURVEY BULLETIN 979-A Manganese Deposits of Western Utah By MAX D. CRITTENDEN, JR. , MANGANESE DEPOSITS OF UTAH, PART 1 GEOLOGICAL SURVEY BULLETIN 979-A A report on known deposits west of the lllth meridian * UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 19S1 UNITED STATES DEPARTMENT OF THE INTERIOR Oscar L. Chapman, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. - Price 50 cents (paper cover) CONTENTS Fag* Abstract.__________________________________________________________ 1 Introduction._._____.__________----_______-______-_--_------.__-__ 1 History of mining and production__.._______.______.___.__-___-_____ 2 Occurrence and age of the deposits_________-_____-_.-__-__-_-__--_- 6 Mineralogy _--____._____---_--_---_------------------------------- 7 Descriptions of the manganese minerals....____.__--_____-__-..__ 8 Oxides...___-__.--_--------___-_-_.-- . _ 8 Carbonates.___-____.__-____________-_-___-----_--------__ 9 Silicate.,_ _____-----_____--__-_______-_---___-__--___._--. 9 Relative stability and manganese content______--_----------_----_ 10 Oxidation and enrichment._____________________________________ 10 Classification and origin of the deposits....______.__._____---.___.-_-_ 11 General discussion_____________________________________________ 11 Syngenetic deposits_-_--____-----_--------------_-------__-_-.- 13 Bedded depositS-__________-_____._____..__________________ 13 Spring -

Download the Scanned
164 THE AMERICAN MINERAI,OGIST TABLE 3 Tlaln ro ssow Mnrnoo or Cer,cur,rfioN oF Axer,ns (SeeWi,nkeltabellen, pp. 18, 19 & 19a). Mlneral Etggttrslte Elements Do:1.272 I€t, Symb, lg Po: 010449 pq qo: .7940 lcp lgq lc so:989982 o I 0 0 01044e oaoosz I p+I 969897 0 e80346 e8es82 I 2 017609 030103 028058nrF,osR I 020085O200R5 qqo :te lpo p lg@:lgtanp lgsin 9 lg cos 9 I sn @ owQ qqo :19 tan p lrom 8 lrom 8 trcm 5 lrom I 3-6:4-7 020467 992858 972381 5802', 017591 56018', 017601 990364 979605 989233 38 42 000741 45 30 000749 007973 988573 980595 50 14 039485 68 04 039490 LISTS OF THE ORTIIORIIOMBIC MINERALS INCLUDED IN GOLDSCIIMIDT'S WINKELTABELLEN. Eocen T. WEONNY. WASh. ington, D. C.-As the prism zone is on the whole most cha.racteristicof orthor- hombic crystals, it has seemeddesirable to arrange the minerals of this system in the order of increasins values of axis a, Onrsonsolmrc MTNERALS ac Page a c Page Uranophanite......0.311.01 355 Tooaz. .....0.53 0.95 346 Polycrasite (Poly- Puiherite..........0.53l.l7 274 kras)...........0.35 0.31 27r Phosohosiderite....0.53 0.88 266 Euxenite ...0.36 0.30 r37 .Iordinite ...0.54 l.oz 191 Molybdite....:....0.39 0,47 243 Yttrobantalite......0.54 1.13 371 Columbite ..0.40 0.36 101 Rammelsbergite....0.54 - 291 Oanneroedite (An- Samarskite.........0.550.52 309 ner<idit).........0.400.36 45 Struvite ....0.55 0.62 332 Flinkite . -

Petrology of Mn Carbonate–Silicate Rocks from the Gangpur Group, India
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by eprints@NML Journal of Asian Earth Sciences 25 (2005) 773–780 www.elsevier.com/locate/jaes Petrology of Mn carbonate–silicate rocks from the Gangpur Group, India B.K. Mohapatraa,*, B. Nayakb aRegional Research Laboratory (CSIR), Bhubaneswar 751 013, India bNational Metallurgical Laboratory (CSIR), Jamshedpur 831 007, India Received 11 April 2003; revised 6 February 2004; accepted 21 July 2004 Abstract Metamorphosed Mn carbonate–silicate rocks with or without oxides (assemblage I) and Mn silicate–oxide rocks with minor Mn carbonate (assemblage II) occur as conformable lenses within metapelites and metacherts of the Precambrian Gangpur Group, India. The petrology of the carbonate minerals: rhodochrosite, kutnahorite, and calcite that occur in these two assemblages is reported. Early stabilisation of spessartine, aegirine, quartz, and carbonates (in a wide solid solution range) was followed by pyroxmangite, tephroite, rhodonite through decarbonation reactions. Subsequently, jacobsite, hematite, braunite, hollandite and hausmannite have formed by decarbonation–oxidation processes during prograde metamorphism. Textural characteristics and chemical composition of constituent phases suggest that the mineral assemblages reflect a complex ! w relationship between protolithic composition, variation of XCO2 ( 0.2 to 0.3) and oxygen fugacity. A variation of XCO2 and fO2 would imply internal buffering of pore fluids through mineral reactions that produced diverse assemblages in the carbonate bearing manganiferous rocks. A minor change in temperature (from around 400 to 450 8C) does not appear to have had any major influence on the formation of different mineral associations. q 2004 Elsevier Ltd. -

A Deposit of Manganese Ore in Wyoming
A DEPOSIT OF MANGANESE ORE IN WYOMING. By EDWARD L. JONES, Jr. INTRODUCTION. Few manganese deposits are known in Wyoming, but one deposit in the Laramie Mountains that was reported to the United States Geological Survey was visited by the writer on October 5, 1917. At that time the deposit was being exploited by the Poverty Mining Co., of Laramie, and 200 tons of ore containing about 40 per cent of manganese was on the dumps, although no ore had been shipped. The deposit is opened by a tunnel and a drift 190 feet in total length, which connect with a shaft 25 feet deep. Six claims constitute the group, which was located April 11,1916. GEOGRAPHY. The deposit of the Poverty Mining Co. lies on a gently sloping mesa on the western flank of the Laramie Mountains, near the head of Sheep Creek, at an altitude of approximately 8,000 feet above sea level. It is accessible from Medicine Bow, on the Union Pacific Railroad, by a fair wagon road 38 miles long. Near the deposit Sheep Creek has eroded in the mesa a channel 250 feet deep, which 'affords a measure of the relief. The rainfall is moderate, and the vegetation consists principally of grasses and small shrubs. Water level has not been reached in the workings. GEOLOGY. The core of the Laramie Mountains is a coarse-grained red granite of pre-Cambrian age, but flanking it on the west side is a series of limestones and sandstones which range in age from Carboniferous to Cretaceous. These rocks underlie the mesa toward Medicine Bow. -
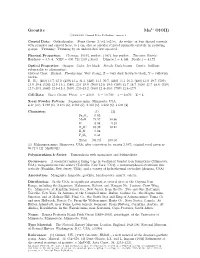
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

GUIDE MINERALS and ROCKS
GUIDE to the MINERALS and ROCKS of Minnesota by GEORGE M. SCHWARTZ and GEORGE A. THIEL DEPARTMENT OF GEOLOGY and MINNESOTA GEOLOGICAL SURVEY UNIVERSITY OF MINNESOTA Guide to the Minerals and Rocks of Minnesota by G. M. Schwartz, Director, Minnesota Geological Survey George A. Thiel, Chairman, Department of Geology University of Minnesota Introduction The minerals and rocks of Minnesota serve as the basis of many im portant industries. They supply the parent materials for our many different types of soils; they contain our vital underground water supplies; and they furnish the raw materials for extensive mineral industries. Many people are interested in the rocks and minerals which they find in road-cuts, in excavations, on their farms, and on their vacation trips. Unusual specimens arouse the curiosity of the finder. Some specimens have peculiar shapes, some have attractive colors and others appear to contain ores of economic value: Many such specimens are received each year at the Department of Geology and the Minnesota Geological Survey of the University of Minnesota. The specimens are generally accompanied by a request for information in regard to the composition, manner of formation, and commercial value of the rock or mineral involved. In response to an increasing number of inquiries from individuals and from schools, this pamphlet was prepared to summarize, as far as possible in nontechnical terms, the different kinds of rocks and minerals found in Minnesota and to describe them so that the amateur collector, the school teacher, the boy and girl scout and other interested persons can identify them. In a pamphlet of this size it is impossible to outline in detail the almost infinite varieties of the common rocks and minerals. -

Bulletin of the Mineral Research and Exploration
Bull. Min. Res. Exp. (2018) 156: 137-150 BULLETIN OF THE MINERAL RESEARCH AND EXPLORATION Foreign Edition 2018 156 ISSN: 0026-4563 CONTENTS Holocene activity of the Orhaneli Fault based on palaoseismological data, Bursa, NW Anatolia Bulletin of the Mineral .......................Volkan ÖZAKSOY, Hasan ELMACI, Selim ÖZALP, Meryem KARA and Tamer Y. DUMAN / Research Article 1 The neotectonics of NE Gaziantep: The Bozova and Halfeti strike-slip faults and their relationships with blind thrusts, Turkey ......................................................................................................... Nuray ùAHBAZ and Gürol SEYøTOöLU / Research Article 17 Neotectonic and morphotectonic characteristics of the Elmali basin and near vicinities .............................................................................................................ùule GÜRBOöA and Özgür AKTÜRK / Research Article 43 Syn-sedimentary deformation structures in the Early Miocene lacustrine deposits, the basal limestone unit, Bigadiç basin (BalÕkesir, Turkey) ..................................................... Calibe KOÇ-TAùGIN, øbrahim TÜRKMEN and Cansu DøNøZ-AKARCA / Research Article 69 The effect of submarine thermal springs of Do÷anbey Cape (Seferihisar - øzmir) on foraminifer, ostracod and mollusc assemblages .................................. Engin MERøÇ, øpek F. BARUT, Atike NAZøK, Niyazi AVùAR, M. Baki YOKEù, Mustafa ERYILMAZ, ........................................ Fulya YÜCESOY-ERYILMAZ, Erol KAM, Bora SONUVAR and Feyza DøNÇER / Research Article