Bulletin of the Mineral Research and Exploration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JAN Iia 20U T6T1/V
IN REPLY REFER TO: UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY WASHINGTON 25. D.C. November 19, 1956 AEC-193/7 Mr. Robert D. Nininger Assistant Director for Exploration Division of Bav Materials U* S. Atomic Energy Commission Washington 25, D. C, Dear Bobs Transmitted herewith are three copies of TEI-622, "The crystal chemistry and mineralogy of vanadium," by Ho-ward T. Evans, Jr. Me are asking Mr. Hosted to approve our plan to publish this report as a chapter of a Geological Survey professional paper on miner alogy and geochemistry of the ores of the Colorado Plateau. Aelrnovledg- ment of AEC sponsorship will be made in the introductory chapter* Sincerely yours, r ^ O U—— TV , Z^*i—w«__ ™~ W. H. Bradley Chief Geoldigist .JAN iia 20U T6T1/V Geology and 8$i:aeralQgy This document consists of k-2 pages* Series A» Howard T. Erans, Jr, Trace Elements Investigations Report 622 This preliminary report is distributed without editorial and technical review for conformity with official standards and nomenclature. It is not for public inspection or quotation* *This report concerns work done on behalf of the Division of Raw Materials of the U. S« Atomic Energy Commission* - TEI-622 AHD MIHERALQ6T Distribution (Series A) Ro. of copies Atomic Energy Commission, Washington .»**«»..*»..«*»..««..*... 2 Division of Rs¥ Materials, Albuquerque ,...****.*.«.»*.....*.. 1 Division of Raw Materials, Austin »«,..«.»...»*.»...*«..*...«» 1 Diylsion of Raw Materials, Butte *«*,.»».*..,*...».»......*.*. 1 Division of Raw Materials, Casper ............a............... 1 Division of Raw Ifeterials, Denver ,........»...».....«.,.*..... 1 Division of Raw Materials, Ishpeming .................a....... 1 Division of Raw Materials, Pnoenix ...a.....,....*........*... 1 Division of Eaw Materials, Rapid City ....................... -

Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3447–3454, March 1999 Colloquium Paper This paper was presented at the National Academy of Sciences colloquium ‘‘Geology, Mineralogy, and Human Welfare,’’ held November 8–9, 1998 at the Arnold and Mabel Beckman Center in Irvine, CA. Manganese oxide minerals: Crystal structures and economic and environmental significance JEFFREY E. POST Department of Mineral Sciences, Smithsonian Institution, Washington, DC 20560-0119 ABSTRACT Manganese oxide minerals have been used ronmentally relevant insights into certain types of interactions for thousands of years—by the ancients for pigments and to between these systems and potentially serve as long-term clarify glass, and today as ores of Mn metal, catalysts, and monitors of changes within a system. battery material. More than 30 Mn oxide minerals occur in a As ores, Mn oxides have been exploited since ancient times. wide variety of geological settings. They are major components In particular, pyrolusite (MnO2) was prized as a pigment and of Mn nodules that pave huge areas of the ocean floor and for its ability to remove the green tint imparted by iron to glass bottoms of many fresh-water lakes. Mn oxide minerals are (3). By the mid-19th century Mn was an essential component ubiquitous in soils and sediments and participate in a variety in steel making, as a deoxidizer and desulfurizer and for of chemical reactions that affect groundwater and bulk soil making hard-steel alloys. Mn oxides are the predominant ore composition. Their typical occurrence as fine-grained mix- minerals in most of today’s commercially important Mn de- tures makes it difficult to study their atomic structures and posits, commonly formed by weathering of Mn-rich carbonates crystal chemistries. -

The Crystal Structure of Ramsdellite from Pirika Mine
Title The Crystal Structure of Ramsdellite from Pirika Mine Author(s) Miura, Hiroyuki; Kudou, Haruhiko; Choi, Jai Ho; Hariya, Yu Citation 北海道大学理学部紀要, 22(4), 611-617 Issue Date 1990-08 Doc URL http://hdl.handle.net/2115/36769 Type bulletin (article) File Information 22_4_p611-617.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Jour. Fac. SeL, Hokkaido Univ., Ser. IV, vol. 22, no. 4, Aug., 1990, pp. 611 -61 7. THE CRYSTAL STRUCTURE OF RAMSDELLITE FROM PIRIKA MINE by Hiroyuki Miura, Haruhiko Kudou: Jai Ho Choi and Yu Hariya (with 3 text-figures and 4 tables) Abstract Chemical composition and crystal structure of ramsdellite from Pirika mine, Hokkaido, Japan were studied. Chemical composition, determined by EPMA, shows that ramsdellite is pure manganese oxide and does not contain any other element. The crystallographic data, obtained by Rietveld analysis, indicate that ramsdellite has orthorhombic symmetry. Space group is Pnma and unit cell parameters are: a = 4.513Cl) A, b = 9.264 Cl) A and c = 2.859Cl)A . The structure consists of MnOs double chains CBystrom, 1950). There are 4 symmetry-dependent MnOs octahe dra in an unit cell and 2 symmetry- independent oxygen atoms in an MnOs octahedron. The difference of inter-atomic distances between Mn-OCl) and Mn -O(2) is larger than other manga nese dioxide minerals. The distortion of MnOs octahedron is quite similar to that of groutite Ca - MnOOH). These data suggest that ramsdellite was formed by oxidation of groutite. Introduction There are several kinds of manganese dioxide minerals such as pyrolusite, hoi· landite, cryptomelane, nsutite, birnessite and ramsdellite. -

Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan
Title Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan Author(s) Hariya, Yu Citation Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and mineralogy, 10(4), 641-702 Issue Date 1961-03 Doc URL http://hdl.handle.net/2115/35921 Type bulletin (article) File Information 10(4)_641-702.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP MKNERALOGICAL STCIDXES ON MANGANESE g]bXCbXgDE ,AND HYDROXIDE MENERALS gN ffE(OKKAI'DO, JAPAN By Yku IEIARIyA (With 29 Text Figures, 17 Tables and 3 Plates) Contribution from the Department of Geology and Mineralogy, Faculty of Seience, Hokkaido University No. 825 CONTENTS I. Introduction,.,..,,.,,.,,.,,,,.,.,,...,,,...,,.,.,,.,.,.,,,.,,., 642 II. Geoteetonic constitution of the Island of Hokkaido ,.,..,.,,.,... 643 1. Geographieal diseribution of mang4nese deposits in Hokkaido . , 644 A. South-western Tegion .,..,.,.,.,....,,.....,..,,,,,.....,.. 644 1) Western sub-province ..,.,.,.,....,,.,,...,.,,..,..,..... 644 a) Palaeozoic deposits .,..,.,.,...,..,..,,.,.,.,,,,,..,.,.,. 645 b) Neogene Tertiary deposits .,,,.,,.,...,.,.,...,.,,.,.,., 645 2) Eastern sub-province.,..,.,.,.,...,,..,,...,.,.,,,..,.,.,. 646 B. Axia} zone.,.,,.,..,.,.,..,...,,,.....,..,,,....,....,...... 646 C. North-eastern region,.,,,.,..,,,,.,.,,,.,,...,.,,,,.,.,.,.,, 647 D. Quaternary volcanie zones .,.,.,,,.,...,..,.,..,.,,.,..,.-・・ 647 2. 0n the respeetive manganese minerals of the above described deposits ......,..,..,..,.,,...,.,.,,,,,..,.,.,.,..,..,....... -

Coupled Substitutions in Natural Mno(OH) Polymorphs: Infrared Spectroscopic Investigation
minerals Article Coupled Substitutions in Natural MnO(OH) Polymorphs: Infrared Spectroscopic Investigation Nikita V. Chukanov 1,2,*, Dmitry A. Varlamov 3 , Igor V. Pekov 2, Natalia V. Zubkova 2, Anatoly V. Kasatkin 4 and Sergey N. Britvin 5 1 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow Region, Russia 2 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia; [email protected] (I.V.P.); [email protected] (N.V.Z.) 3 Institute of Experimental Mineralogy Russian Academy of Sciences, 142432 Chernogolovka, Russia; [email protected] 4 Fersman Mineralogical Museum of the Russian Academy of Sciences, Leninsky Prospekt 18-2, 119071 Moscow, Russia; [email protected] 5 Crystallography Department, Institute of Earth Sciences, St. Petersburg State University, University emb. 7/9, 199034 St. Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Solid solutions involving natural Mn3+O(OH) polymorphs, groutite, manganite, and feitknechtite are characterized and discussed based on original and literature data on the chemical composition, powder and single-crystal X-ray diffraction, and middle-range IR absorption spectra of these minerals. It is shown that manganite forms two kinds of solid-solution series, in which 4+ 3+ intermediate members have the general formulae (i) (Mn , Mn )O(OH,O), with pyrolusite as the 4+ 3+ 2+ Mn O2 end-member, and (ii) (Mn ,M )O(OH, H2O), where M = Mn or Zn. In Zn-substituted 2+ 3+ Citation: Chukanov, N.V.; Varlamov, manganite from Kapova Cave, South Urals, Russia, the Zn :Mn ratio reaches 1:1 (the substitution 3+ 2+ − D.A.; Pekov, I.V.; Zubkova, N.V.; of Mn with Zn is accompanied by the coupled substitution of OH with H2O). -

THE MINERALOGY of the Mapiml' MINING DISTRICT, DURANGO
The mineralogy of the Mapimí mining district, Durango, Mexico Item Type text; Dissertation-Reproduction (electronic) Authors Hoffmann, Victor Joseph 1935- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 05/10/2021 08:35:35 Link to Item http://hdl.handle.net/10150/565169 THE MINERALOGY OF THE MAPIMl' MINING DISTRICT, DURANGO, MEXICO by Victor Joseph Hoffmann A Dissertation Submitted to the Faculty of the DEPARTMENT OF GEOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 19 6 8 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE I hereby recommend that this dissertation prepared under my direction by _________ Victor Joseph Hoffmann____________________ entitled The Mineralogy of the Mapimi Mining District,______ Durango, Mexico______________________________ be accepted as fulfilling the dissertation requirement of the degree of Doctor of Philosophy_______________________________ ____________ ‘7/2 __________________ Dissertation Director^/ Date z / ~ After inspection of the final copy of the dissertation, the following members of the Final Examination Committee concur in its approval and recommend its acceptance:* f , A> Q ~/ w n n rT 2.7, 7 / f / 7 u Z Z /9<$7 •fs---------- - ' -------7 This approval and acceptance is contingent on the candidate's adequate performance and defense of this dissertation at the final oral examination. The inclusion of this sheet bound into the library copy of the dissertation is evidence of satisfactory performance at the final examination. -

SOME STABILITY RELATIONS in the SYSTEM Mn-Oz-Hzo at 25" and ONE ATMOSPHERE TOTAL PRESSURE Ownrc Bnrcrnn, H Art:Ard,U N'irtersity, Cambridge, M Assachwsetts
THE AMERICAN MINERALOGIST, VOL. 50, SEPTEMBER, 1965 SOME STABILITY RELATIONS IN THE SYSTEM Mn-Oz-HzO AT 25" AND ONE ATMOSPHERE TOTAL PRESSURE Ownrc Bnrcrnn, H art:ard,U n'irtersity, Cambridge, M assachwsetts. ABsrRAcr Stability relations in the system Mn-OrHzO rvere investigated at 25o C. and one atmo- sphere total pressure.Seven compounds, Mn(OH)2, MnsOr, y-Mn:Os, r-MnOOH, 6-MnOz, 7-MnO2, and hydrohausmannite s'ere synthesized under controlled conditions of Eh and pH. Hydrohausmannite was found to be a mixture of MnsO+ and/or l-Mn:Or and p-MnOOH rather than a single phase.The name feitknechtite is proposed for naturally oc- curring B-MnOOH. Free energy of formation data were obtained for the first six com- -132.2 -133.3 pounds listed. They are respectively: -147.14 kcal., -306.2 kcal , kcal., kcal., - 108.3 kcai, and - 109 1 kcal. Eh-pH diagrams were constructed to shorv stability relations among the phases in the system Mn-OrHzO. Good correspondencewas found between stability relations observed in the laboratory and those observed in natural occur- rencesof manganeseoxides. A model is describedfor the supergeneoxidation of rhodochro- site. INrnooucrroN Manganese,a transition metal, is capableof existing in a number of differentoxidation states (2f,3+,4+,6+,7+). The 2f and 4* oxidation states are the lowest and highest that have been observedin nature, although the ephemeralexistence of other statesis conceivable. Commonly, manganeseoxide minerals contain more than one oxidation state of manganese,and a continuousseries between 2* and 4* is ob- served in the average oxidation state of manganesein supergeneoxides (whether a 3f oxidationstate actually occursin the solid state or is real- ized through an equal distribution of 2* and 4f statesremains a moot question). -

Supplementary Information Mitigating Effect of Organic Matter on the in Vivo Toxicity of Metal Oxide Nanoparticles in the Marine Environment
Electronic Supplementary Material (ESI) for Environmental Science: Nano. This journal is © The Royal Society of Chemistry 2018 Supplementary Information Mitigating effect of organic matter on the in vivo toxicity of metal oxide nanoparticles in the marine environment Seta Noventa*a, Darren Rowea and Tamara Gallowaya *corresponding author: email: [email protected], tel: 01392 263436 a College of Life and Environmental Sciences, University of Exeter, EX4 4QD, Exeter UK, This file includes additional information about: Primary physico-chemical properties of the test-NPs and methods used for the characterization Methods for the characterization of the behaviour of the test-NPs Primer sequences, qPCR conditions and performance MnO2 NP aggregation kinetics MnO2 NP ingestion TEM-EDS study of cellular internalization of MnO2 NPs TEM-EDS analyses of electron dense particles suspected of being cellular internalized BSA coated MnO2 NPs Abbreviations: NPs: nanoparticles; TEM: Transmission Electron Microscopy; BET: Branauer, Emmett and Teller; SEM: Scanning Electron Microscope; EDS: Energy Dispersive X-ray Spectroscopy; pHIEP: pH at the isoelectric point; DIW: deionised water; ASW: artificial seawater; Cyt c: Cytochrome c. Primary physico-chemical properties of the test-NPs and methods used for the characterization The primary physico-chemical proprieties of the test NPs, ZnO NPs, and MnO2 NPs, are summarized in Table S1. Data was retrieved from official reports (for ZnO NPs only 1) and supplemented with data performed at the NERC Facility for Environmental Nanoscience Analysis and Characterisation (FENAC, Birmingham, UK). The description of the methods used and more details about the results obtained are reported below. Table S1. Primary physico-chemical proprieties of the test NPs, ZnO NPs and MnO2 NPs. -

Neutron and Synchrotron X-Ray Diffraction Study of the Structures and Dehydration Behaviors of Ramsdellite and “Groutellite”
American Mineralogist, Volume 89, pages 969–975, 2004 Neutron and synchrotron X-ray diffraction study of the structures and dehydration behaviors of ramsdellite and “groutellite” JEFFREY E. POST1,* AND PETER J. HEANEY2 1Department of Mineral Sciences, Smithsonian Institution, Washington, D.C. 20560-0119, U.S.A. 2 Department of Geosciences, 309 Deike, Pennsylvania State University, University Park, Pennsylvania 16802, U.S.A. ABSTRACT The crystal structure of ramsdellite, MnO2, was refined using time-of-flight powder neutron dif- fraction data and the Rietveld method in order to assess the effects of reduction in cathodic battery materials. For the first time, we present a refined structure for “groutellite,” a heretofore poorly char- 4+ 3+ acterized phase with ideal formula (Mn0.5Mn0.5)O1.5(OH)0.5. “Groutellite” is generated synthetically as an intermediate compound during the reduction of ramsdellite to groutite (MnOOH), and it also occurs as an intergrowth in certain natural specimens of ramsdellite. The Jahn-Teller distortions in “groutellite” are confined to thea -c plane, and they result in a 6.8% unit-cell volume increase relative to ramsdellite. The Mn–O bond lengths refined for “groutellite” are consistent with the replacement of half of the Mn4+ and O2- in ramsdellite by Mn3+ and (OH)–, respectively. In addition, the high- temperature behaviors of ramsdellite and “groutellite” were investigated by temperature-resolved synchrotron powder X-ray diffraction from 298 to 720 K. Rietveld refinements revealed a gradual thermal expansion of the groutellite structure to ∼450 K. At higher temperatures, the unit-cell volume gradually decreased, primarily as a result of a decrease in c. -

Supporting Information
ORE Open Research Exeter TITLE Dissolution and bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: an in vivo study with oyster embryos AUTHORS Noventa, S; Hacker, C; Rowe, D; et al. JOURNAL Nanotoxicology DEPOSITED IN ORE 29 August 2018 This version available at http://hdl.handle.net/10871/33846 COPYRIGHT AND REUSE Open Research Exeter makes this work available in accordance with publisher policies. A NOTE ON VERSIONS The version presented here may differ from the published version. If citing, you are advised to consult the published version for pagination, volume/issue and date of publication Supporting Information Dissolution and Bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: an in vivo study with oyster embryos Seta Noventa*a, Christian Hackerb, Darren Rowea, Christine Elgyc and Tamara Gallowaya *corresponding author: email: [email protected], tel: 01392 263436 a College of Life and Environmental Sciences, University of Exeter, EX4 4QD, Exeter UK, b College of Life and Environmental Sciences, Bioimaging Centre, University of Exeter, EX4 4QD, Exeter UK c Department of Geography, Earth and Environmental Sciences, Facility for Environmental Nanoscience Analysis and Characterisation, University of Birmingham, Edgbaston, Birmingham, UK This file includes additional information about: • Primary physico-chemical properties of the test-NPs • Primer sequences, qPCR conditions and performance • TEM-EDS study of biological fate of MnO2 NPs • Methods for the characterization of the behaviour of the test-NPs • Results of the Cytochrome c oxidation assay carried out on ZnO and CeO2 NP dispersions in deionized water Abbreviations: NPs: nanoparticles; TEM: Transmission Electron Microscopy; BET: Branauer, Emmett and Teller; EELS: Electron Energy Loss Spectroscopy; SEM: Scanning Electron Microscope; EDS: Energy Dispersive X-ray Spectroscopy; DIW: deionized water; ASW: artificial seawater; Cyt c: Cytochrome c; pHIEP: pH at the isoelectric point. -
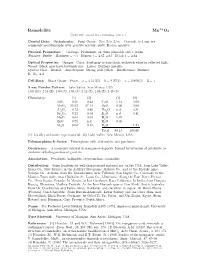
Ramsdellite Mn O2 C 2001-2005 Mineral Data Publishing, Version 1
4+ Ramsdellite Mn O2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. Crystals, to 1 cm, are commonly pseudomorphs after groutite crystals; platy, fibrous, massive. Physical Properties: Cleavage: Prominent, on three pinacoids and a prism. Tenacity: Brittle. Hardness = ∼3 D(meas.) = 4.65–4.83 D(calc.) = 4.84 Optical Properties: Opaque. Color: Steel-gray to iron-black; yellowish white in reflected light. Streak: Black, may have brownish tint. Luster: Brilliant metallic. Optical Class: Biaxial. Anisotropism: Strong; pale yellow. Bireflectance: Distinct. R1–R2: n.d. Cell Data: Space Group: P bnm. a = 4.533(5) b = 9.27(1) c = 2.866(5) Z = 4 X-ray Powder Pattern: Lake Valley, New Mexico, USA. 4.08 (10), 2.53 (8), 1.60 (7), 1.64 (6), 2.13 (5), 1.88 (5), 1.46 (5) Chemistry: (1) (2) (1) (2) SiO2 0.36 0.42 CaO 1.12 0.00 MnO2 95.02 97.14 BaO 0.00 0.00 Al2O3 0.72 0.48 Na2O n.d. 0.31 Fe2O3 0.22 0.34 K2O n.d. 0.41 + MnO 0.00 0.00 H2O 1.39 − ZnO 0.25 n.d. H2O 0.05 MgO 0.00 0.12 H2O 1.24 Total 99.13 100.46 (1) Locality unknown; type material. (2) Lake Valley, New Mexico, USA. Polymorphism & Series: Trimorphous with akhtenskite and pyrolusite. Occurrence: A secondary mineral in manganese deposits, formed by inversion of pyrolusite or oxidative dehydrogenation of groutite. Association: Pyrolusite, hollandite, cryptomelane, coronadite. -

Depositional Modes of Manganese Oxides at Artillery Peak, Mohave County, Arizona
UNIVERSITY OF NEVADA, RENO Depositional Modes of Manganese Oxides at Artillery Peak, Mohave County, Arizona A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Geology Sean Derby 4/15/2012 By Sean F. Derby Dr. Tommy B. Thompson/Thesis Advisor May 2012 THE GRADUATE SCHOOL We recommend that the thesis prepared under our supervision by SEAN DERBY entitled Depositional Modes Of Manganese Oxides At Artillery Peak, Mohave County, Arizona be accepted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Tommy Thompson, Advisor John Mccormack, Committee Member Scott Bassett, Graduate School Representative Marsha H. Read, Ph. D., Dean, Graduate School May, 2012 i Abstract ~ Economically significant manganese oxides deposits within Southwestern North America share many commonalities regarding alteration, depositional environment/mode, relative age, and mineralogy. These deposits can be broadly divided into two categories; terrestrial vein – type deposits (volcanogenic or hydrothermal) and terrestrial sedimentary rock – hosted stratiform – type deposits. Original depositional environments for stratiform deposits are interpreted as soils and bogs with hydrothermal enrichment or as a detrital component of a hot springs system redeposited into lacustrian basins. Vein – type manganese is believed to be a product of mineralizing aqueous fluids undergoing Eh – pH changes during ascent through near surface host rock or sediments (Roy, 1992). Manganese ions are naturally mobile at the earth’s surface due to weathering, and manganese is the second most abundant transition metal at the earth surface. Reworking of manganese readily occurs due to late fluid influences within a wide range of temperature and chemical settings.