Neutron and Synchrotron X-Ray Diffraction Study of the Structures and Dehydration Behaviors of Ramsdellite and “Groutellite”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JAN Iia 20U T6T1/V
IN REPLY REFER TO: UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY WASHINGTON 25. D.C. November 19, 1956 AEC-193/7 Mr. Robert D. Nininger Assistant Director for Exploration Division of Bav Materials U* S. Atomic Energy Commission Washington 25, D. C, Dear Bobs Transmitted herewith are three copies of TEI-622, "The crystal chemistry and mineralogy of vanadium," by Ho-ward T. Evans, Jr. Me are asking Mr. Hosted to approve our plan to publish this report as a chapter of a Geological Survey professional paper on miner alogy and geochemistry of the ores of the Colorado Plateau. Aelrnovledg- ment of AEC sponsorship will be made in the introductory chapter* Sincerely yours, r ^ O U—— TV , Z^*i—w«__ ™~ W. H. Bradley Chief Geoldigist .JAN iia 20U T6T1/V Geology and 8$i:aeralQgy This document consists of k-2 pages* Series A» Howard T. Erans, Jr, Trace Elements Investigations Report 622 This preliminary report is distributed without editorial and technical review for conformity with official standards and nomenclature. It is not for public inspection or quotation* *This report concerns work done on behalf of the Division of Raw Materials of the U. S« Atomic Energy Commission* - TEI-622 AHD MIHERALQ6T Distribution (Series A) Ro. of copies Atomic Energy Commission, Washington .»**«»..*»..«*»..««..*... 2 Division of Rs¥ Materials, Albuquerque ,...****.*.«.»*.....*.. 1 Division of Raw Materials, Austin »«,..«.»...»*.»...*«..*...«» 1 Diylsion of Raw Materials, Butte *«*,.»».*..,*...».»......*.*. 1 Division of Raw Materials, Casper ............a............... 1 Division of Raw Ifeterials, Denver ,........»...».....«.,.*..... 1 Division of Raw Materials, Ishpeming .................a....... 1 Division of Raw Materials, Pnoenix ...a.....,....*........*... 1 Division of Eaw Materials, Rapid City ....................... -
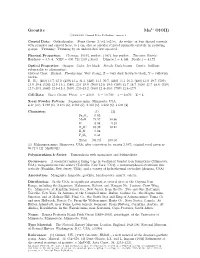
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

GUIDE MINERALS and ROCKS
GUIDE to the MINERALS and ROCKS of Minnesota by GEORGE M. SCHWARTZ and GEORGE A. THIEL DEPARTMENT OF GEOLOGY and MINNESOTA GEOLOGICAL SURVEY UNIVERSITY OF MINNESOTA Guide to the Minerals and Rocks of Minnesota by G. M. Schwartz, Director, Minnesota Geological Survey George A. Thiel, Chairman, Department of Geology University of Minnesota Introduction The minerals and rocks of Minnesota serve as the basis of many im portant industries. They supply the parent materials for our many different types of soils; they contain our vital underground water supplies; and they furnish the raw materials for extensive mineral industries. Many people are interested in the rocks and minerals which they find in road-cuts, in excavations, on their farms, and on their vacation trips. Unusual specimens arouse the curiosity of the finder. Some specimens have peculiar shapes, some have attractive colors and others appear to contain ores of economic value: Many such specimens are received each year at the Department of Geology and the Minnesota Geological Survey of the University of Minnesota. The specimens are generally accompanied by a request for information in regard to the composition, manner of formation, and commercial value of the rock or mineral involved. In response to an increasing number of inquiries from individuals and from schools, this pamphlet was prepared to summarize, as far as possible in nontechnical terms, the different kinds of rocks and minerals found in Minnesota and to describe them so that the amateur collector, the school teacher, the boy and girl scout and other interested persons can identify them. In a pamphlet of this size it is impossible to outline in detail the almost infinite varieties of the common rocks and minerals. -

Bulletin of the Mineral Research and Exploration
Bull. Min. Res. Exp. (2018) 156: 137-150 BULLETIN OF THE MINERAL RESEARCH AND EXPLORATION Foreign Edition 2018 156 ISSN: 0026-4563 CONTENTS Holocene activity of the Orhaneli Fault based on palaoseismological data, Bursa, NW Anatolia Bulletin of the Mineral .......................Volkan ÖZAKSOY, Hasan ELMACI, Selim ÖZALP, Meryem KARA and Tamer Y. DUMAN / Research Article 1 The neotectonics of NE Gaziantep: The Bozova and Halfeti strike-slip faults and their relationships with blind thrusts, Turkey ......................................................................................................... Nuray ùAHBAZ and Gürol SEYøTOöLU / Research Article 17 Neotectonic and morphotectonic characteristics of the Elmali basin and near vicinities .............................................................................................................ùule GÜRBOöA and Özgür AKTÜRK / Research Article 43 Syn-sedimentary deformation structures in the Early Miocene lacustrine deposits, the basal limestone unit, Bigadiç basin (BalÕkesir, Turkey) ..................................................... Calibe KOÇ-TAùGIN, øbrahim TÜRKMEN and Cansu DøNøZ-AKARCA / Research Article 69 The effect of submarine thermal springs of Do÷anbey Cape (Seferihisar - øzmir) on foraminifer, ostracod and mollusc assemblages .................................. Engin MERøÇ, øpek F. BARUT, Atike NAZøK, Niyazi AVùAR, M. Baki YOKEù, Mustafa ERYILMAZ, ........................................ Fulya YÜCESOY-ERYILMAZ, Erol KAM, Bora SONUVAR and Feyza DøNÇER / Research Article -

Minerals of Arizona Report
MINERALS OF ARIZONA by Frederic W. Galbraith and Daniel J. Brennan THE ARIZONA BUREAU OF MINES Price One Dollar Free to Residents of Arizona Bulletin 181 1970 THE UNIVERSITY OF ARIZONA TUCSON TABLE OF CONT'ENTS EIements .___ 1 FOREWORD Sulfides ._______________________ 9 As a service about mineral matters in Arizona, the Arizona Bureau Sulfosalts ._. .___ __ 22 of Mines, University of Arizona, is pleased to reprint the long-standing booklet on MINERALS OF ARIZONA. This basic journal was issued originally in 1941, under the authorship of Dr. Frederic W. Galbraith, as Simple Oxides .. 26 a bulletin of the Arizona Bureau of Mines. It has moved through several editions and, in some later printings, it was authored jointly by Dr. Gal Oxides Containing Uranium, Thorium, Zirconium .. .... 34 braith and Dr. Daniel J. Brennan. It now is being released in its Fourth Edition as Bulletin 181, Arizona Bureau of Mines. Hydroxides .. .. 35 The comprehensive coverage of mineral information contained in the bulletin should serve to give notable and continuing benefits to laymen as well as to professional scientists of Arizona. Multiple Oxides 37 J. D. Forrester, Director Arizona Bureau of Mines Multiple Oxides Containing Columbium, February 2, 1970 Tantaum, Titanium .. .. .. 40 Halides .. .. __ ____ _________ __ __ 41 Carbonates, Nitrates, Borates .. .... .. 45 Sulfates, Chromates, Tellurites .. .. .. __ .._.. __ 57 Phosphates, Arsenates, Vanadates, Antimonates .._ 68 First Edition (Bulletin 149) July 1, 1941 Vanadium Oxysalts ...... .......... 76 Second Edition, Revised (Bulletin 153) April, 1947 Third Edition, Revised 1959; Second Printing 1966 Fourth Edition (Bulletin 181) February, 1970 Tungstates, Molybdates.. _. .. .. .. 79 Silicates ... -

Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3447–3454, March 1999 Colloquium Paper This paper was presented at the National Academy of Sciences colloquium ‘‘Geology, Mineralogy, and Human Welfare,’’ held November 8–9, 1998 at the Arnold and Mabel Beckman Center in Irvine, CA. Manganese oxide minerals: Crystal structures and economic and environmental significance JEFFREY E. POST Department of Mineral Sciences, Smithsonian Institution, Washington, DC 20560-0119 ABSTRACT Manganese oxide minerals have been used ronmentally relevant insights into certain types of interactions for thousands of years—by the ancients for pigments and to between these systems and potentially serve as long-term clarify glass, and today as ores of Mn metal, catalysts, and monitors of changes within a system. battery material. More than 30 Mn oxide minerals occur in a As ores, Mn oxides have been exploited since ancient times. wide variety of geological settings. They are major components In particular, pyrolusite (MnO2) was prized as a pigment and of Mn nodules that pave huge areas of the ocean floor and for its ability to remove the green tint imparted by iron to glass bottoms of many fresh-water lakes. Mn oxide minerals are (3). By the mid-19th century Mn was an essential component ubiquitous in soils and sediments and participate in a variety in steel making, as a deoxidizer and desulfurizer and for of chemical reactions that affect groundwater and bulk soil making hard-steel alloys. Mn oxides are the predominant ore composition. Their typical occurrence as fine-grained mix- minerals in most of today’s commercially important Mn de- tures makes it difficult to study their atomic structures and posits, commonly formed by weathering of Mn-rich carbonates crystal chemistries. -

The Crystal Structure of Ramsdellite from Pirika Mine
Title The Crystal Structure of Ramsdellite from Pirika Mine Author(s) Miura, Hiroyuki; Kudou, Haruhiko; Choi, Jai Ho; Hariya, Yu Citation 北海道大学理学部紀要, 22(4), 611-617 Issue Date 1990-08 Doc URL http://hdl.handle.net/2115/36769 Type bulletin (article) File Information 22_4_p611-617.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Jour. Fac. SeL, Hokkaido Univ., Ser. IV, vol. 22, no. 4, Aug., 1990, pp. 611 -61 7. THE CRYSTAL STRUCTURE OF RAMSDELLITE FROM PIRIKA MINE by Hiroyuki Miura, Haruhiko Kudou: Jai Ho Choi and Yu Hariya (with 3 text-figures and 4 tables) Abstract Chemical composition and crystal structure of ramsdellite from Pirika mine, Hokkaido, Japan were studied. Chemical composition, determined by EPMA, shows that ramsdellite is pure manganese oxide and does not contain any other element. The crystallographic data, obtained by Rietveld analysis, indicate that ramsdellite has orthorhombic symmetry. Space group is Pnma and unit cell parameters are: a = 4.513Cl) A, b = 9.264 Cl) A and c = 2.859Cl)A . The structure consists of MnOs double chains CBystrom, 1950). There are 4 symmetry-dependent MnOs octahe dra in an unit cell and 2 symmetry- independent oxygen atoms in an MnOs octahedron. The difference of inter-atomic distances between Mn-OCl) and Mn -O(2) is larger than other manga nese dioxide minerals. The distortion of MnOs octahedron is quite similar to that of groutite Ca - MnOOH). These data suggest that ramsdellite was formed by oxidation of groutite. Introduction There are several kinds of manganese dioxide minerals such as pyrolusite, hoi· landite, cryptomelane, nsutite, birnessite and ramsdellite. -

Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan
Title Mineralogical Studies on Manganese Dioxide and Hydroxide Minerals in Hokkaido, Japan Author(s) Hariya, Yu Citation Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and mineralogy, 10(4), 641-702 Issue Date 1961-03 Doc URL http://hdl.handle.net/2115/35921 Type bulletin (article) File Information 10(4)_641-702.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP MKNERALOGICAL STCIDXES ON MANGANESE g]bXCbXgDE ,AND HYDROXIDE MENERALS gN ffE(OKKAI'DO, JAPAN By Yku IEIARIyA (With 29 Text Figures, 17 Tables and 3 Plates) Contribution from the Department of Geology and Mineralogy, Faculty of Seience, Hokkaido University No. 825 CONTENTS I. Introduction,.,..,,.,,.,,.,,,,.,.,,...,,,...,,.,.,,.,.,.,,,.,,., 642 II. Geoteetonic constitution of the Island of Hokkaido ,.,..,.,,.,... 643 1. Geographieal diseribution of mang4nese deposits in Hokkaido . , 644 A. South-western Tegion .,..,.,.,.,....,,.....,..,,,,,.....,.. 644 1) Western sub-province ..,.,.,.,....,,.,,...,.,,..,..,..... 644 a) Palaeozoic deposits .,..,.,.,...,..,..,,.,.,.,,,,,..,.,.,. 645 b) Neogene Tertiary deposits .,,,.,,.,...,.,.,...,.,,.,.,., 645 2) Eastern sub-province.,..,.,.,.,...,,..,,...,.,.,,,..,.,.,. 646 B. Axia} zone.,.,,.,..,.,.,..,...,,,.....,..,,,....,....,...... 646 C. North-eastern region,.,,,.,..,,,,.,.,,,.,,...,.,,,,.,.,.,.,, 647 D. Quaternary volcanie zones .,.,.,,,.,...,..,.,..,.,,.,..,.-・・ 647 2. 0n the respeetive manganese minerals of the above described deposits ......,..,..,..,.,,...,.,.,,,,,..,.,.,.,..,..,....... -

Coupled Substitutions in Natural Mno(OH) Polymorphs: Infrared Spectroscopic Investigation
minerals Article Coupled Substitutions in Natural MnO(OH) Polymorphs: Infrared Spectroscopic Investigation Nikita V. Chukanov 1,2,*, Dmitry A. Varlamov 3 , Igor V. Pekov 2, Natalia V. Zubkova 2, Anatoly V. Kasatkin 4 and Sergey N. Britvin 5 1 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow Region, Russia 2 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia; [email protected] (I.V.P.); [email protected] (N.V.Z.) 3 Institute of Experimental Mineralogy Russian Academy of Sciences, 142432 Chernogolovka, Russia; [email protected] 4 Fersman Mineralogical Museum of the Russian Academy of Sciences, Leninsky Prospekt 18-2, 119071 Moscow, Russia; [email protected] 5 Crystallography Department, Institute of Earth Sciences, St. Petersburg State University, University emb. 7/9, 199034 St. Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Solid solutions involving natural Mn3+O(OH) polymorphs, groutite, manganite, and feitknechtite are characterized and discussed based on original and literature data on the chemical composition, powder and single-crystal X-ray diffraction, and middle-range IR absorption spectra of these minerals. It is shown that manganite forms two kinds of solid-solution series, in which 4+ 3+ intermediate members have the general formulae (i) (Mn , Mn )O(OH,O), with pyrolusite as the 4+ 3+ 2+ Mn O2 end-member, and (ii) (Mn ,M )O(OH, H2O), where M = Mn or Zn. In Zn-substituted 2+ 3+ Citation: Chukanov, N.V.; Varlamov, manganite from Kapova Cave, South Urals, Russia, the Zn :Mn ratio reaches 1:1 (the substitution 3+ 2+ − D.A.; Pekov, I.V.; Zubkova, N.V.; of Mn with Zn is accompanied by the coupled substitution of OH with H2O). -

THE MINERALOGY of the Mapiml' MINING DISTRICT, DURANGO
The mineralogy of the Mapimí mining district, Durango, Mexico Item Type text; Dissertation-Reproduction (electronic) Authors Hoffmann, Victor Joseph 1935- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 05/10/2021 08:35:35 Link to Item http://hdl.handle.net/10150/565169 THE MINERALOGY OF THE MAPIMl' MINING DISTRICT, DURANGO, MEXICO by Victor Joseph Hoffmann A Dissertation Submitted to the Faculty of the DEPARTMENT OF GEOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 19 6 8 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE I hereby recommend that this dissertation prepared under my direction by _________ Victor Joseph Hoffmann____________________ entitled The Mineralogy of the Mapimi Mining District,______ Durango, Mexico______________________________ be accepted as fulfilling the dissertation requirement of the degree of Doctor of Philosophy_______________________________ ____________ ‘7/2 __________________ Dissertation Director^/ Date z / ~ After inspection of the final copy of the dissertation, the following members of the Final Examination Committee concur in its approval and recommend its acceptance:* f , A> Q ~/ w n n rT 2.7, 7 / f / 7 u Z Z /9<$7 •fs---------- - ' -------7 This approval and acceptance is contingent on the candidate's adequate performance and defense of this dissertation at the final oral examination. The inclusion of this sheet bound into the library copy of the dissertation is evidence of satisfactory performance at the final examination. -

Descriptions of Northeast Asia Metallogenic Belts
Northeast Asia Metallogenc Belt Descriptions – May 5, 2004 DESCRIPTIONS OF NORTHEAST ASIA METALLOGENIC BELTS By Sergey M. Rodionov1, Alexander A. Obolenskiy2, Elimir G. Distanov2, Gombosuren Badarch3, Gunchin Dejidmaa4, Duk Hwan Hwang5, Alexander I.Khanchuk6, Masatsugu Ogasawara7, Warren J. Nokleberg8, Leonid M. Parfenov9, Andrei V. Prokopiev9, Zhan V. Seminskiy10, Alexander P. Smelov9, Hongquan Yan11, Yuriy V. V. Davydov9, Valeriy Yu. Fridovskiy12 , Gennandiy N. Gamyanin9, Ochir Gerel13, Alexei V. Kostin9, Sergey A. Letunov14, Xujun Li11, Valeriy M. Nikitin12, Vladimir V. Ratkin6, Vladimir I. Shpikerman15, Sadahisa Sudo7, Vitaly I. Sotnikov2, Alexander V. Spiridonov14, Vitaly A. Stepanov16, Fengyue Sun11, Jiapeng Sun11, Weizhi Sun11, Valeriy M. Supletsov9, Vladimir F. Timofeev9, Oleg A. Tyan9, Valeriy G. Vetluzhskikh9, Koji Wakita7, Yakov V. Yakovlev9, and 14 Lydia M. Zorina Edited by Sergey M. Rodionov1, Alexander A. Obolenskiy2, Zhan V. Seminskiy10, Tatiana V. Bounaeva14, and Warren J. Nokleberg8 1 Russian Academy of Sciences, Khabarovsk 2 Russian Academy of Sciences, Novosibirsk 3 Mongolian Academy of Sciences, Ulaanbaatar 4 Mineral Resources Authority of Mongolia, Ulaanbaatar 5 Korean Institute of Geology, Mining, and Mineral Resources, Taejon 6 Russian Academy of Sciences, Vladivostok 7 Geological Survey of Japan/AIST, Tsukuba 8 U.S. Geological Survey, Menlo Park 9 Russian Academy of Sciences, Yakutsk 10 Irkutsk State Technical University, Irkutsk 11 Jilin University, Changchun 12 Yakutian State University, Yakutsk 13 Mongolian University of Science and Technology, Ulaanbaatar 14 Russian Academy of Sciences, Irkutsk 15 Russian Academy of Sciences, Magadan 16 Russian Academy of Sciences, Blagoveschensk 1 Northeast Asia Metallogenc Belt Descriptions – May 5, 2004 Introduction and Companion Studies The metallogenic belts of Northeast Asia are herein synthesized, compiled, described, and interpreted with the use of modern concepts of plate tectonics, terranes and overlap assemblages, and mineral deposit models. -

SOME STABILITY RELATIONS in the SYSTEM Mn-Oz-Hzo at 25" and ONE ATMOSPHERE TOTAL PRESSURE Ownrc Bnrcrnn, H Art:Ard,U N'irtersity, Cambridge, M Assachwsetts
THE AMERICAN MINERALOGIST, VOL. 50, SEPTEMBER, 1965 SOME STABILITY RELATIONS IN THE SYSTEM Mn-Oz-HzO AT 25" AND ONE ATMOSPHERE TOTAL PRESSURE Ownrc Bnrcrnn, H art:ard,U n'irtersity, Cambridge, M assachwsetts. ABsrRAcr Stability relations in the system Mn-OrHzO rvere investigated at 25o C. and one atmo- sphere total pressure.Seven compounds, Mn(OH)2, MnsOr, y-Mn:Os, r-MnOOH, 6-MnOz, 7-MnO2, and hydrohausmannite s'ere synthesized under controlled conditions of Eh and pH. Hydrohausmannite was found to be a mixture of MnsO+ and/or l-Mn:Or and p-MnOOH rather than a single phase.The name feitknechtite is proposed for naturally oc- curring B-MnOOH. Free energy of formation data were obtained for the first six com- -132.2 -133.3 pounds listed. They are respectively: -147.14 kcal., -306.2 kcal , kcal., kcal., - 108.3 kcai, and - 109 1 kcal. Eh-pH diagrams were constructed to shorv stability relations among the phases in the system Mn-OrHzO. Good correspondencewas found between stability relations observed in the laboratory and those observed in natural occur- rencesof manganeseoxides. A model is describedfor the supergeneoxidation of rhodochro- site. INrnooucrroN Manganese,a transition metal, is capableof existing in a number of differentoxidation states (2f,3+,4+,6+,7+). The 2f and 4* oxidation states are the lowest and highest that have been observedin nature, although the ephemeralexistence of other statesis conceivable. Commonly, manganeseoxide minerals contain more than one oxidation state of manganese,and a continuousseries between 2* and 4* is ob- served in the average oxidation state of manganesein supergeneoxides (whether a 3f oxidationstate actually occursin the solid state or is real- ized through an equal distribution of 2* and 4f statesremains a moot question).