Groutite, Hmno2, a New Mineral of the Diaspore-Goethite Group
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diaspore Alo(OH) C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic
Diaspore AlO(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. As crystals, platy on {010} and elongated to acicular along [001], to 40 cm; stalactitic, foliated, scaly; disseminated, massive. Twinning: On {021}, to form heart-shaped twins or pseudohexagonal aggregates; on {061}, uncommon. Physical Properties: Cleavage: {010} perfect, {110} distinct, {100} in traces. Fracture: Conchoidal. Tenacity: Very brittle. Hardness = 6.5–7 D(meas.) = 3.2–3.5 D(calc.) = 3.380 Optical Properties: Transparent to translucent. Color: White, pale gray, colorless, greenish gray, brown, pale yellow, pink, lilac; color may vary with viewing direction in the same specimen, may show a color change from brownish green in daylight to raspberry pink in artificial light; colorless in thin section. Luster: Adamantine, vitreous, pearly on cleavage faces. Optical Class: Biaxial (+). Pleochroism: In thick plates, may be reddish brown to reddish violet; grayish green to green. Orientation: X = c; Y = b; Z = a. Dispersion: r< v,weak. Absorption: Z > Y > X, seen on strongly colored specimens. α = 1.682–1.706 β = 1.705–1.725 γ = 1.730–1.752 2V(meas.) = 84◦–86◦ Cell Data: Space Group: P bnm. a = 4.4007(6) b = 9.4253(13) c = 2.8452(3) Z = 4 X-ray Powder Pattern: Springfield, Massachusetts, USA. 3.99 (100), 2.317 (56), 2.131 (52), 2.077 (49), 1.633 (43), 2.558 (30), 1.480 (20) Chemistry: (1) (2) (1) (2) SiO2 0.42 Fe2O3 0.18 Al2O3 84.44 84.98 H2O 14.99 15.02 Total 100.03 100.00 (1) Kossoi Brod, Russia. -

Nonsteady-State Dissolution of Goethite and Hematite in Response to Ph Jumps: the Role of Adsorbed Fe (III)
Water-Rock Interactions, Ore Deposits, and Environmental Geochemistry: A Tribute to David A. Crerar © The Geochemical Society, Special Publication No.7, 2002 Editors: Roland Hellmann and Scott A Wood Nonsteady-state dissolution of goethite and hematite in response to pH jumps: the role of adsorbed Fe (III) SHERRY D. SAMSON* AND CARRICK M. EGGLESTON Department of Geology and Geophysics, University of Wyoming, Laramie, WY 82071 USA * Author to whom correspondence should be addressed ([email protected]). Present address: Department of Geological Sciences, University of Colorado, Boulder CO 80309 USA Abstract-Dissolution transients following downward pH jumps to pH 1from a variety of higher pH values during dissolution in a mixed-flow reactor contain information about dissolution processes and mechanisms. Despite more than an order of magnitude difference in steady-state dissolution rates, the transients for goethite (a.-FeOOH) (this study) and hematite (a.-Fe203) (Samson and Eggleston, 1998) are similar in their pH dependence and relaxation times. After a pH jump, the time required to reach a new steady state is 40 to 50 hours. The amount of excess Fe released in the transients (defined as the amount of Fe released in excess of that released in an equivalent length of time during steady-state dissolution at pH 1) increases with in- creasing initial (i.e., pre-jump) pH, and is dependent on initial pH in a manner similar to the pH dependence of Fe3+ adsorption to other oxides. We suggest that the excess Fe released in the transients is derived from partial dissolution or depolymerization of the iron (hydr)oxide at pH ~ I and the transition of such Fe into the adsorbed state on the mineral surface. -

The Iron Oxides Structure, Properties, Reactions, Occurrence and Uses
R.M.Cornell U. Schwertmann The Iron Oxides Structure, Properties, Reactions, Occurrence and Uses Weinheim • New York VCH Basel • Cambridge • Tokyo Contents 1 Introduction to the iron oxides 1 2 Crystal structure 7 2.1 General 7 2.2 Iron oxide structures 7 2.2.1 Close packing of anion layers 10 2.2.2 Linkages of octahedra or tetrahedra 12 2.3 Structures of the individual iron oxides 14 2.3.1 The oxide hydroxides 14 2.3.1.1 Goethite a-FeOOH 14 2.3.1.2 Lepidocrocite y-FeO(OH) 16 2.3.1.3 Akaganeite ß-FeO(OH) and schwertmannite Fe16016(OH)y(S04)z • n H20 18 2.3.1.4 5-FeOOH and 8'-FeOOH (feroxyhyte) 20 2.3.1.5 High pressure FeOOH 21 2.3.1.6 Ferrihydrite 22 2.3.2 The hydroxides 24 2.3.2.1 Bernalite Fe(OH)3 • nH20 24 2.3.2.2 Fe(OH)2 25 2.3.2.3 Green rusts 25 2.3.3 The oxides 26 2.3.3.1 Haematite a-Fe203 26 2.3.3.2 Magnetite Fe304 28 2.3.3.3 Maghemite y-Fe203 30 2.3.3.4 Wüstite Fe^O 31 2.4 The Fe-Ti oxide System 33 3 Cation Substitution 35 3.1 General 35 3.2 Goethite 38 3.2.1 AI Substitution 38 3.2.1.1 Synthetic goethites 38 3.2.1.2 Natural goethites 43 3.2.2 Other substituting cations 43 3.3 Haematite 48 3.4 Other Fe oxides 50 VIII Contents 4 Crystal morphology and size 53 4.1 General 53 4.1.1 Crystal growth 53 4.1.2 Crystal morphology 55 4.1.3 Crystal size 57 4.2 The iron oxides 58 4.2.1 Goethite 59 4.2.1.1 General 59 4.2.1.2 Domainic character 64 4.2.1.3 Twinning 66 4.2.1.4 Effect of additives on goethite morphology 68 4.2.2 Lepidocrocite 70 4.2.3 Akaganeite and schwertmannite 71 4.2.4 Ferrihydrite 73 4.2.5 Haematite 74 4.2.6 Magnetite 82 4.2.7 -

The Structure and Vibrational Spectroscopy of Cryolite, Na3alf6 Cite This: RSC Adv., 2020, 10, 25856 Stewart F
RSC Advances PAPER View Article Online View Journal | View Issue The structure and vibrational spectroscopy of cryolite, Na3AlF6 Cite this: RSC Adv., 2020, 10, 25856 Stewart F. Parker, *a Anibal J. Ramirez-Cuesta b and Luke L. Daemenb Cryolite, Na3[AlF6], is essential to commercial aluminium production because alumina is readily soluble in molten cryolite. While the liquid state has been extensively investigated, the spectroscopy of the solid state has been largely ignored. In this paper, we show that the structure at 5 K is the same as that at Received 31st May 2020 room temperature. We use a combination of infrared and Raman spectroscopies together with inelastic Accepted 1st July 2020 neutron scattering (INS) spectroscopy. The use of INS enables access to all of the modes of Na3[AlF6], DOI: 10.1039/d0ra04804f including those that are forbidden to the optical spectroscopies. Our spectral assignments are supported rsc.li/rsc-advances by density functional theory calculations of the complete unit cell. Introduction In view of the technological importance of cryolite, we have Creative Commons Attribution 3.0 Unported Licence. carried out a comprehensive spectroscopic investigation and 1 Cryolite, Na3[AlF6], occurs naturally as a rare mineral. Histori- report new infrared and Raman spectra over extended temper- cally, it was used as a source of aluminium but this has been ature and spectral ranges and the inelastic neutron scattering superseded by bauxite (a mixture of the Al2O3 containing (INS) spectrum. The last of these is observed for the rst time minerals boehmite, diaspore and gibbsite), largely because of and enables access to all of the modes of Na3[AlF6]. -

Minerals of the San Luis Valley and Adjacent Areas of Colorado Charles F
New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/22 Minerals of the San Luis Valley and adjacent areas of Colorado Charles F. Bauer, 1971, pp. 231-234 in: San Luis Basin (Colorado), James, H. L.; [ed.], New Mexico Geological Society 22nd Annual Fall Field Conference Guidebook, 340 p. This is one of many related papers that were included in the 1971 NMGS Fall Field Conference Guidebook. Annual NMGS Fall Field Conference Guidebooks Every fall since 1950, the New Mexico Geological Society (NMGS) has held an annual Fall Field Conference that explores some region of New Mexico (or surrounding states). Always well attended, these conferences provide a guidebook to participants. Besides detailed road logs, the guidebooks contain many well written, edited, and peer-reviewed geoscience papers. These books have set the national standard for geologic guidebooks and are an essential geologic reference for anyone working in or around New Mexico. Free Downloads NMGS has decided to make peer-reviewed papers from our Fall Field Conference guidebooks available for free download. Non-members will have access to guidebook papers two years after publication. Members have access to all papers. This is in keeping with our mission of promoting interest, research, and cooperation regarding geology in New Mexico. However, guidebook sales represent a significant proportion of our operating budget. Therefore, only research papers are available for download. Road logs, mini-papers, maps, stratigraphic charts, and other selected content are available only in the printed guidebooks. Copyright Information Publications of the New Mexico Geological Society, printed and electronic, are protected by the copyright laws of the United States. -

High-Temperature Thermomagnetic Properties of Vivianite Nodules
EGU Journal Logos (RGB) Open Access Open Access Open Access Advances in Annales Nonlinear Processes Geosciences Geophysicae in Geophysics Open Access Open Access Natural Hazards Natural Hazards and Earth System and Earth System Sciences Sciences Discussions Open Access Open Access Atmospheric Atmospheric Chemistry Chemistry and Physics and Physics Discussions Open Access Open Access Atmospheric Atmospheric Measurement Measurement Techniques Techniques Discussions Open Access Open Access Biogeosciences Biogeosciences Discussions Open Access Open Access Clim. Past, 9, 433–446, 2013 Climate www.clim-past.net/9/433/2013/ Climate doi:10.5194/cp-9-433-2013 of the Past of the Past © Author(s) 2013. CC Attribution 3.0 License. Discussions Open Access Open Access Earth System Earth System Dynamics Dynamics Discussions High-temperature thermomagnetic properties of Open Access Open Access vivianite nodules, Lake El’gygytgyn, Northeast RussiaGeoscientific Geoscientific Instrumentation Instrumentation P. S. Minyuk1, T. V. Subbotnikova1, L. L. Brown2, and K. J. Murdock2 Methods and Methods and 1North-East Interdisciplinary Scientific Research Institute, Far East Branch of the Russian AcademyData Systems of Sciences, Data Systems Magadan, Russia Discussions Open Access 2 Open Access Department of Geosciences, University of Massachusetts, Amherst, USA Geoscientific Geoscientific Correspondence to: P. S. Minyuk ([email protected]) Model Development Model Development Received: 7 September 2012 – Published in Clim. Past Discuss.: 9 October 2012 Discussions Revised: 15 January 2013 – Accepted: 15 January 2013 – Published: 19 February 2013 Open Access Open Access Hydrology and Hydrology and Abstract. Vivianite, a hydrated iron phosphate, is abun- 1 Introduction Earth System Earth System dant in sediments of Lake El’gygytgyn, located in the Anadyr Mountains of central Chukotka, northeastern Rus- Sciences Sciences sia (67◦300 N, 172◦050 E). -

Iidentilica2tion and Occurrence of Uranium and Vanadium Identification and Occurrence of Uranium and Vanadium Minerals from the Colorado Plateaus
IIdentilica2tion and occurrence of uranium and Vanadium Identification and Occurrence of Uranium and Vanadium Minerals From the Colorado Plateaus c By A. D. WEEKS and M. E. THOMPSON A CONTRIBUTION TO THE GEOLOGY OF URANIUM GEOLOGICAL S U R V E Y BULL E TIN 1009-B For jeld geologists and others having few laboratory facilities.- This report concerns work done on behalf of the U. S. Atomic Energy Commission and is published with the permission of the Commission. UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1954 UNITED STATES DEPARTMENT OF THE- INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan. Director Reprint, 1957 For sale by the Superintendent of Documents, U. S. Government Printing Ofice Washington 25, D. C. - Price 25 cents (paper cover) CONTENTS Page 13 13 13 14 14 14 15 15 15 15 16 16 17 17 17 18 18 19 20 21 21 22 23 24 25 25 26 27 28 29 29 30 30 31 32 33 33 34 35 36 37 38 39 , 40 41 42 42 1v CONTENTS Page 46 47 48 49 50 50 51 52 53 54 54 55 56 56 57 58 58 59 62 TABLES TABLE1. Optical properties of uranium minerals ______________________ 44 2. List of mine and mining district names showing county and State________________________________________---------- 60 IDENTIFICATION AND OCCURRENCE OF URANIUM AND VANADIUM MINERALS FROM THE COLORADO PLATEAUS By A. D. WEEKSand M. E. THOMPSON ABSTRACT This report, designed to make available to field geologists and others informa- tion obtained in recent investigations by the Geological Survey on identification and occurrence of uranium minerals of the Colorado Plateaus, contains descrip- tions of the physical properties, X-ray data, and in some instances results of chem- ical and spectrographic analysis of 48 uranium arid vanadium minerals. -

Redox Trapping of Arsenic During Groundwater Discharge in Sediments from the Meghna Riverbank in Bangladesh
Redox trapping of arsenic during groundwater discharge in sediments from the Meghna riverbank in Bangladesh S. Dattaa,b, B. Maillouxc, H.-B. Jungd, M. A. Hoquee, M. Stutea,c, K. M. Ahmede, and Y. Zhenga,d,1 aLamont-Doherty Earth Observatory of Columbia University, 61 Route 9W, Palisades, New York, NY 10964; bKansas State University, Department of Geology, Manhattan, KS 66506; cBarnard College, Department of Environmental Sciences, New York, NY 10027; dQueens College, School of Earth and Environmental Sciences, City University of New York, Flushing, New York, NY 11367; and eUniversity of Dhaka, Department of Geology, Dhaka, 1000 Bangladesh Communicated by Charles H. Langmuir, Harvard University, Cambridge, MA, July 30, 2009 (received for review September 5, 2007) Groundwater arsenic (As) is elevated in the shallow Holocene Results aquifers of Bangladesh. In the dry season, the shallow groundwa- Aquifers in the GBMD. The Ganges and Brahmaputra rivers coalesce ter discharges to major rivers. This process may influence the northwest of Dhaka and then join the Meghna River south of chemistry of the river and the hyporheic zone sediment. To assess Dhaka before flowing into the Bay of Bengal (see Fig. 1). Bang- the fate of As during discharge, surface (0–5 cm) and subsurface ladesh is less than 10 m above sea level, except for the uplifted (1–3 m) sediment samples were collected at 9 sites from the bank Pleistocene terraces, the Chittagong Hills, and the hilly area in the of the Meghna River along a transect from its northern source northeast. The sandy, unconsolidated Pleistocene to Holocene (25° N) to the Bay of Bengal (22.5° N). -

Petrography and Geochemistry of the Banded Iron Formation of the Gangfelum Area, Northeastern Nigeria
Earth Science Research; Vol. 7, No. 1; 2018 ISSN 1927-0542 E-ISSN 1927-0550 Published by Canadian Center of Science and Education Petrography and Geochemistry of the Banded Iron Formation of the Gangfelum Area, Northeastern Nigeria Anthony Temidayo Bolarinwa1 1 Department of Geology, University of Ibadan, Ibadan, Nigeria Correspondence: Anthony Temidayo Bolarinwa, Department of Geology, University of Ibadan, Ibadan, Nigeria. E-mail: [email protected] Received: September 15, 2016 Accepted: October 3, 2016 Online Published: October 3, 2017 doi:10.5539/esr.v7n1p25 URL: https://doi.org/10.5539/esr.v7n1p25 Abstract The Gangfelum Banded Iron Formation (BIF) is located within the basement complex of northeastern Nigeria. It is characterized by alternate bands of iron oxide and quartz. Petrographic studies show that the BIF consist mainly of hematite, goethite subordinate magnetite and accessory minerals including rutile, apatite, tourmaline and zircon. Chemical data from inductively coupled plasma optical emission spectrometer (ICP-OES) and inductively coupled plasma mass spectrometer (ICP-MS) show that average Fe2O3(t) is 53.91 wt.%. The average values of Al2O3 and CaO are 1.41 and 0.05 wt.% respectively, TiO2 and MnO are less than 0.5 wt. % each. The data suggested that the BIF is the oxide facies type. Trace element concentrations of Ba (67-332 ppm), Ni (28-35 ppm), Sr (13-55 ppm) and Zr (16-25 ppm) in the Gangfelum BIF are low and similar to the Maru and Muro BIF in northern Nigeria and also the Algoma iron formation from North America, the Orissa iron oxide facies of India and the Itabirite from Minas Gerais in Brazil. -
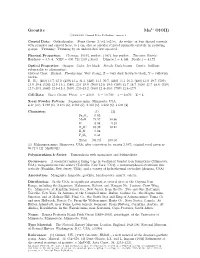
Groutite Mn3+O(OH) C 2001-2005 Mineral Data Publishing, Version 1
Groutite Mn3+O(OH) c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m2/m2/m. As wedge- or lens-shaped crystals with rounded and curved faces, to 1 cm; also as acicular striated prismatic crystals, in radiating groups. Twinning: Twinning by an undescribed law reported. Physical Properties: Cleavage: {010}, perfect; {100}, less perfect. Tenacity: Brittle. Hardness = 3.5–4 VHN = 630–711 (100 g load). D(meas.) = 4.144 D(calc.) = 4.172 Optical Properties: Opaque. Color: Jet-black. Streak: Dark brown. Luster: Brilliant submetallic to adamantine. Optical Class: Biaxial. Pleochroism: Very strong; X = very dark brown to black; Y = yellowish brown. R1–R2: (400) 13.7–22.0, (420) 13.4–21.3, (440) 13.3–20.7, (460) 13.1–20.2, (480) 13.0–19.7, (500) 13.0–19.4, (520) 12.9–19.1, (540) 12.9–19.0, (560) 12.8–19.0, (580) 12.7–18.7, (600) 12.7–18.6, (620) 12.7–18.5, (640) 12.6–18.3, (660) 12.5–18.2, (680) 12.4–18.0, (700) 12.4–17.9 Cell Data: Space Group: P bnm. a = 4.560 b = 10.700 c = 2.870 Z = 4 X-ray Powder Pattern: Sagamore mine, Minnesota, USA. 4.17 (10), 2.798 (6), 2.675 (6), 2.369 (6), 2.303 (5), 1.692 (5), 1.603 (4) Chemistry: (1) (2) Fe2O3 0.02 MnO 79.97 80.66 O 8.94 9.10 + H2O 10.39 10.24 − H2O 0.04 P2O5 0.34 Total [99.70] 100.00 (1) Mahnomen mine, Minnesota, USA; after correction for quartz 2.39%, original total given as 99.71% (2) MnO(OH). -

Visible-To-Near-Infrared Spectral Variability of Hydrated Sulfates and Candidate Mars Landing Sites
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Winter 2018 Visible-to-Near-Infrared Spectral Variability of Hydrated Sulfates and Candidate Mars Landing Sites: Implications for the Mastcam- Z Investigation on NASA’s Mars-2020 Rover Mission Darian Dixon Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Dixon, Darian, "Visible-to-Near-Infrared Spectral Variability of Hydrated Sulfates and Candidate Mars Landing Sites: Implications for the Mastcam-Z Investigation on NASA’s Mars-2020 Rover Mission" (2018). WWU Graduate School Collection. 638. https://cedar.wwu.edu/wwuet/638 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. Visible-to-Near-Infrared Spectral Variability of Hydrated Sulfates and Candidate Mars Landing Sites: Implications for the Mastcam-Z Investigation on NASA’s Mars-2020 Rover Mission By Darian Dixon Accepted in Partial Completion of the Requirements for the Degree Master of Science Kathleen L. Kitto, Dean of the Graduate School ADVISORY COMMITTEE Chair, Dr. Melissa Rice Dr. Pete Stelling Dr. Michael Kraft MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. -

Properties of Synthetic Goethites and Their Effect on Sulfate Adsorption Munoz, Miguel A., Ph.D
Order Number 8824578 Properties of synthetic goethites and their effect on sulfate adsorption Munoz, Miguel A., Ph.D. The Ohio State University, 1988 Copyright ©1988by Munoz, Miguel A. All rights reserved. UMI 300 N. Zeeb Rd. Ann Arbor, MI 48106 PLEASE NOTE: In all cases this material has been filmed in the best possible way from the available copy. Problems encountered with this document have been identified here with a check mark •/ . 1. Glossy photographs or pages. 2. Colored illustrations, paper or print • 3. Photographs with dark background _____ 4. Illustrations are poor copy ____ 5. Pages with black marks, not original copy ^ 6. Print shows through as there is text on both sides of page ______ 7. Indistinct, broken or small print on several pages _______ 8. Print exceeds margin requirements______ 9. Tightly bound copy with print lost in spine _______ 10. Computer printout pages with indistinct print ______ 11. Page(s)___________ lacking when material received, and not available from school or author. 12. Page(s) seem to be missing in numbering only as text follows. 13. Two pages numbered . Text follows. 14. Curling and wrinkled pages S 15. Dissertation contains pages with print at a slant, filmed as received 16. Other PROPERTIES OF SYNTHETIC GOETHITES AND THEIR EFFECT ON SULFATE ADSORPTION DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Miguel A. Munoz, B.S., M.S. The Ohio State University 1988 Dissertation Committee: Approved by Dr. J .M . Bigham Dr. S.J.