Analogy = Computer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -
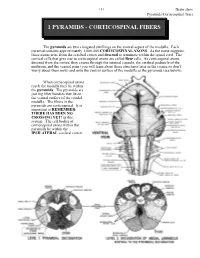
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Brainstem: Structure & Its Mode of Action
Journal of Neurology & Neurophysiology 2021, Vol.12, Issue 3, 521 Opinion Brainstem: Structure & Its Mode of action Karthikeyan Rupani Research Fellow, Tata Medical Centre, India. Corresponding Author* The brainstem is exceptionally little, making up around as it were 2.6 percent of the brain's add up to weight. It has the basic parts of directing cardiac, and Rupani K, respiratory work, making a difference to control heart rate and breathing rate. Research Fellow, Tata Medical Centre, India; It moreover gives the most engine and tactile nerve supply to the confront and E-mail: [email protected] neck by means of the cranial nerves. Ten sets of cranial nerves come from the brainstem. Other parts incorporate the direction of the central apprehensive Copyright: 2021 Rupani K. This is an open-access article distributed under the framework and the body's sleep cycle. It is additionally of prime significance terms of the Creative Commons Attribution License, which permits unrestricted within the movement of engine and tangible pathways from the rest of the use, distribution, and reproduction in any medium, provided the original author brain to the body, and from the body back to the brain. These pathways and source are credited. incorporate the corticospinal tract (engine work), the dorsal column-medial lemniscus pathway and the spinothalamic tract [3]. The primary part of the brainstem we'll consider is the midbrain. The midbrain Received 01 March 2021; Accepted 15 March 2021; Published 22 March 2021 (too known as the mesencephalon) is the foremost prevalent of the three districts of the brainstem. It acts as a conduit between the forebrain over and the pons and cerebellum underneath. -

The Strain Rates in the Brain, Brainstem, Dura, and Skull Under Dynamic Loadings
Mathematical and Computational Applications Article The Strain Rates in the Brain, Brainstem, Dura, and Skull under Dynamic Loadings Mohammad Hosseini-Farid 1,2,* , MaryamSadat Amiri-Tehrani-Zadeh 3, Mohammadreza Ramzanpour 1, Mariusz Ziejewski 1 and Ghodrat Karami 1 1 Department of Mechanical Engineering, North Dakota State University, Fargo, ND 58104, USA; [email protected] (M.R.); [email protected] (M.Z.); [email protected] (G.K.) 2 Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN 55905, USA 3 Department of Computer Science, North Dakota State University, Fargo, ND 58104, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-7012315859 Received: 7 March 2020; Accepted: 5 April 2020; Published: 7 April 2020 Abstract: Knowing the precise material properties of intracranial head organs is crucial for studying the biomechanics of head injury. It has been shown that these biological tissues are significantly rate-dependent; hence, their material properties should be determined with respect to the range of deformation rate they experience. In this paper, a validated finite element human head model is used to investigate the biomechanics of the head in impact and blast, leading to traumatic brain injuries (TBI). We simulate the head under various directions and velocities of impacts, as well as helmeted and unhelmeted head under blast shock waves. It is demonstrated that the strain rates for the brain 1 are in the range of 36 to 241 s− , approximately 1.9 and 0.86 times the resulting head acceleration under impacts and blast scenarios, respectively. The skull was found to experience a rate in the range 1 of 14 to 182 s− , approximately 0.7 and 0.43 times the head acceleration corresponding to impact and blast cases. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

What to Expect After Having a Subarachnoid Hemorrhage (SAH) Information for Patients and Families Table of Contents
What to expect after having a subarachnoid hemorrhage (SAH) Information for patients and families Table of contents What is a subarachnoid hemorrhage (SAH)? .......................................... 3 What are the signs that I may have had an SAH? .................................. 4 How did I get this aneurysm? ..................................................................... 4 Why do aneurysms need to be treated?.................................................... 4 What is an angiogram? .................................................................................. 5 How are aneurysms repaired? ..................................................................... 6 What are common complications after having an SAH? ..................... 8 What is vasospasm? ...................................................................................... 8 What is hydrocephalus? ............................................................................... 10 What is hyponatremia? ................................................................................ 12 What happens as I begin to get better? .................................................... 13 What can I expect after I leave the hospital? .......................................... 13 How will the SAH change my health? ........................................................ 14 Will the SAH cause any long-term effects? ............................................. 14 How will my emotions be affected? .......................................................... 15 When should -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Differentiation of the Cerebellum 2463
Development 128, 2461-2469 (2001) 2461 Printed in Great Britain © The Company of Biologists Limited 2001 DEV1660 Inductive signal and tissue responsiveness defining the tectum and the cerebellum Tatsuya Sato, Isato Araki‡ and Harukazu Nakamura* Department of Molecular Neurobiology, Institute of Development, Aging and Cancer, Seiryo-machi 4-1, Aoba-ku, Sendai 980- 8575, Japan ‡Present address: Department of Neurobiology, University of Heidelberg, Im Neuenheimer Feld 364, D-69120 Heidelberg, Germany *Author for correspondence (e-mail: [email protected]) Accepted 11 April 2001 SUMMARY The mes/metencephalic boundary (isthmus) has an Fgf8b repressed Otx2 expression, but upregulated Gbx2 and organizing activity for mesencephalon and metencephalon. Irx2 expression in the mesencephalon. As a result, Fgf8b The candidate signaling molecule is Fgf8 whose mRNA is completely changed the fate of the mesencephalic alar plate localized in the region where the cerebellum differentiates. to cerebellum. Quantitative analysis showed that Fgf8b Responding to this signal, the cerebellum differentiates in signal is 100 times stronger than Fgf8a signal. Co- the metencephalon and the tectum differentiates in the transfection of Fgf8b with Otx2 indicates that Otx2 is a key mesencephalon. Based on the assumption that strong Fgf8 molecule in mesencephalic generation. We have shown by signal induces the cerebellum and that the Fgf8b signal is RT-PCR that both Fgf8a and Fgf8b are expressed, Fgf8b stronger than that of Fgf8a, we carried out experiments to expression prevailing in the isthmic region. The results all misexpress Fgf8b and Fgf8a in chick embryos. Fgf8a did not support our working hypothesis that the strong Fgf8 signal affect the expression pattern of Otx2, Gbx2 or Irx2. -

Meninges Ventricles And
Meninges ,ventricles & CSF Dr.Sanaa Al-Shaarawy Dr. Essam Eldin Salama OBJECTIVES • By the end of the lecture the student should be able to: • Describe the cerebral meninges & list the main dural folds. • Describe the spinal meninges & locate the level of the termination of each of them. • Describe the importance of the subarachnoid space. • List the Ventricular system of the CNS and locate the site of each of them. • Describe the formation, circulation, drainage, and functions of the CSF. • Know some clinical point about the CSF MENINGES • The brain and spinal cord are invested by three concentric membranes ; • The outermost layer is the dura matter. • The middle layer is the arachnoid matter. • The innermost layer is the pia matter. DURA MATER ▪The cranial dura is a two layered tough, fibrous thick membrane that surrounds the brain. ▪It is formed of two layers; periosteal and meningeal. ▪The periosteal layer is attached to the skull. ▪The meningeal layer is folded forming the dural folds : falx cerebri, and tentorium cerebelli. ▪Sensory innervation of the dura is mostly from : meningeal branches of the trigeminal and vagus nerves & C1 to C3(upper cervical Ns.). DURA MATER Folds Two large reflection of dura extend into the cranial cavity : 1.The falx cerebri, In the midline, ▪It is a vertical sickle-shaped sheet of dura, extends from the cranial roof into the great longitudinal fissure between the two cerebral hemispheres. ▪It has an attached border adherent to the skull. ▪And a free border lies above the corpus callosum. DURA MATER Folds 2. A horizontal shelf of dura, The tentorium cerebelli, ▪ It lies between the posterior part of the cerebral hemispheres and the cerebellum. -

NERVOUS SYSTEM هذا الملف لالستزادة واثراء المعلومات Neuropsychiatry Block
NERVOUS SYSTEM هذا الملف لﻻستزادة واثراء المعلومات Neuropsychiatry block. قال تعالى: ) َو َل َق د َخ َل قنَا ا ِْلن َسا َن ِمن ُس ََل َل ة ِ من ِطي ن }12{ ثُ م َجعَ لنَاه ُ نُ ط َفة فِي َق َرا ر م ِكي ن }13{ ثُ م َخ َل قنَا ال ُّن ط َفة َ َع َل َقة َف َخ َل قنَا ا لعَ َل َقة َ ُم ضغَة َف َخ َل قنَا ا ل ُم ضغَة َ ِع َظا ما َف َك َس ونَا ا ل ِع َظا َم َل ح ما ثُ م أَن َشأنَاه ُ َخ ل قا آ َخ َر َفتَبَا َر َك ّللا ُ أَ ح َس ُن ا ل َخا ِل ِقي َن }14{( Resources BRS Embryology Book. Pathoma Book ( IN DEVELOPMENTAL ANOMALIES PART ). [email protected] 1 OVERVIEW A- Central nervous system (CNS) is formed in week 3 of development, during which time the neural plate develops. The neural plate, consisting of neuroectoderm, becomes the neural tube, which gives rise to the brain and spinal cord. B- Peripheral nervous system (PNS) is derived from three sources: 1. Neural crest cells 2. Neural tube, which gives rise to all preganglionic autonomic nerves (sympathetic and parasympathetic) and all nerves (-motoneurons and -motoneurons) that innervate skeletal muscles 3. Mesoderm, which gives rise to the dura mater and to connective tissue investments of peripheral nerve fibers (endoneurium, perineurium, and epineurium) DEVELOPMENT OF THE NEURAL TUBE Neurulation refers to the formation and closure of the neural tube. BMP-4 (bone morphogenetic protein), noggin (an inductor protein), chordin (an inductor protein), FGF-8 (fibroblast growth factor), and N-CAM (neural cell adhesion molecule) appear to play a role in neurulation.