Use the Decision Was Signed on the Same Day As the Completion of the OP Cumulative Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organophosphate Poisoning : a Review
120 Sinha and Sharma Med J Indones Organophosphate poisoning : A review Parmod K. Sinha, Ashok Sharma Abstrak Pestisida organofosfat digunakan secara luas di seluruh dunia. Keracunan oleh bahan ini merupakan masalah kesehatan masyarakat, terutama di negara berkembang. Zat neurotoksik organofosfat merupakan bahan yang dianggap mengancam dalam bidang militer dan terorisme. Mekanisme toksisitas bahan ini adalah dengan cara menghambat asetilkolinesterase yang mengakibatkan menumpuknya neurotransmitor asetilkolin dan terjadi rangsangan terus-menerus pada reseptor asetilkolin pada sistem saraf sentral maupun perifer. Selain krisis kolinergik, organofosfat dapat menimbulkan berbagai sindrom neurologis, baik akut maupun kronik. Sedangkan gejala peralihan ( intermediate) terjadi 1-4 hari setelah krisis kolinergik teratasi. Pengobatan standar terdiri dari reaktivasi asetilkolinesterase dengan antidot golongan oksim (prolidoksim, oksidoksime, HI-6 dan HLo7), dan pengendalian efek biokimia asetilkolin dengan menggunakan atropin. Golongan oksim yang baru HI-6 dan Hlo7 merupakan reaktivator asetilkolinesterase yang lebih cocok dan efektif untuk keracunan akut dan berat dibandingkan dengan prolidoksim dan obidoksim. Penderita yang mendapat pengobatan segera, biasanya dapat sembuh dari toksisitas akut, namun gejala neurologis ikutan dapat saja terjadi. (Med J Indones 2003; 12: 120-6) Abstract Organophosphate pesticides are used extensively worldwide, and poisoning by these agents, particularly in developing nations is a public health problem. Organophosphorous -

Guide No. 1 – October 2020 2/12 the CONCEPT and IMPLEMENTATION of CPA GUIDANCE RESIDUE LEVELS
Cooperation Centre for Scientific Research Relative to Tobacco CORESTA GUIDE N° 1 The Concept and Implementation of CPA Guidance Residue Levels October 2020 Agro-Chemical Advisory Committee CORESTA TECHNICAL GUIDE N° 1 Title: The Concept and Implementation of CPA Guidance Residue Levels Status: Valid Note: This document will be periodically reviewed by CORESTA Document history: Date of review Information July 2003 Version 1 GRL for Pyrethrins () and Terbufos corrected. December 2003 CPA terminology corrected. June 2008 Version 2 – GRLs revised and residue definitions added Provisional GRL of 2.00 ppm for Cyfluthrin to replace previous June 2010 GRL of 0.50 ppm July 2013 Version 3 – GRLs revised October 2013 Note for Maleic Hydrazide revised Version 4 – GRLs revised + clarification that scope of GRLs July 2016 applies predominantly to the production of traditional cigarette tobaccos and GAP associated with their cultivation. June 2018 Fluopyram GRL of 5 ppm added to GRL list Version 5 – Nine new CPAs with GRL added to list. November 2019 Revision of GRLs for Chlorantraniliprole and Indoxacarb. Updated web links. October 2020 Version 6 – Flupyradifurone GRL of 21 ppm added to GRL list. CORESTA Guide No. 1 – October 2020 2/12 THE CONCEPT AND IMPLEMENTATION OF CPA GUIDANCE RESIDUE LEVELS Executive Summary • Guidance Residue Levels (GRLs) are in the remit of the Agro-Chemical Advisory Committee (ACAC) of CORESTA. Their development is a joint activity of all ACAC members, who represent the leaf production, processing and manufacturing sectors of the Tobacco Industry. The concept of GRLs and their implementation are described in this guide. • GRLs provide guidance to tobacco growers and assist with interpretation and evaluation of results from analyses of residues of Crop Protection Agents (CPAs*). -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Quantification of Organophosphorus Pesticides by Quechers and LC
HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS Column: Synergi™ 4 µm Fusion-RP 80 Å, LC Column 50 x 2 mm, Ea Dimensions: 50 x 2 mm ID Order No: 00B-4424-B0 Elution Type: Gradient Eluent A: Add 5mL of 1M ammonium formate to 1L Water Eluent B: Add 5mL of 1M ammonium formate to 1L Methanol Gradient Step No. Time (min) Pct A Pct B Profile: 1 -5 95 5 2 0 95 5 3 2 95 5 4 6 50 50 5 12 10 90 6 14 10 90 7 16 95 5 8 17 95 5 Flow Rate: 250 mL/min Col. Temp.: 25 °C Detection: Tandem Mass Spec (MS-MS) @ (ambient) Detector Info: <a target="_blank" href="https://sciex.com/products/mass-spectrometers?utm_campaign=2019%20application%20search&utm_source=phenomenex&utm_medium=referral">SCIEX</a> Analyst Note: SecurityGuard™ Guard Cartridge System extends column lifetime. - SecurityGuard Cartridges, Fusion-RP 4 x 2.0mm ID, 10/Pk Part No.: AJ0-7556 - Holder Part No.: KJ0-4282 ©2021 Phenomenex Inc. All rights reserved. For more information contact your Phenomenex Representative at [email protected] Phenomenex products are available worldwide. www.phenomenex.com [email protected] Page 1 of 2 HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS ANALYTES: 1 Acephate (MRM: 184/142.8) 41 Diazinon (MRM: 305/169) 2 Acephate (MRM: 184/94.9) 42 Diazinon (MRM: 305/153) 3 Demeton-S-methyl sulfone (MRM: 263/169) 43 Fenthion (MRM: 278.9/169) 4 Demeton-S-methyl sulfone (MRM: 263/109) 44 Fenthion (MRM: 278.9/246.9) 5 Parathion-methyl Methyl parathion (MRM: 263.9/232.1)45 -

How to Reduce Bee Poisoning from Pesticides
PNW 591 December 2006 How to ReduceReduce BeeBee PoisoningPoisoning from pesticides H. Riedl E. Johansen L. Brewer J. Barbour A Pacific Northwest Extension publication Oregon State University • University of Idaho • Washington State University Contents Pollinators are essential to Pacific Northwest agriculture .......................................................................1 Rules to protect bees ..............................................................................................................................1 Causes of bee poisoning in the Pacific Northwest .................................................................................2 Investigating a suspected bee poisoning ................................................................................................2 Signs and symptoms of bee poisoning ...................................................................................................2 Honey bees .................................................................................................................................................... 2 Managed solitary bees ................................................................................................................................... 3 Ways to reduce bee poisoning ...............................................................................................................3 Beekeeper–grower cooperation ..................................................................................................................... 3 What pesticide -

(12) Patent Application Publication (10) Pub. No.: US 2009/0208480 A1 Huang Et Al
US 20090208480A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0208480 A1 Huang et al. (43) Pub. Date: Aug. 20, 2009 (54) LONG HALF-LIFE RECOMBINANT Related U.S. Application Data BUTYRYLCHOLINESTERASE (60) Provisional application No. 60/835,827, filed on Aug. 4, 2006. (76) Inventors: Yue Huang, Vaudreuil-Dorion O O (CA); Harvey Wilgus, Beaconsfield Publication Classification (CA) (51) Int. Cl. A638/46 (2006.01) Correspondence Address: CI2N 9/18 (2006.01) Alan J. Grant A6IP39/00 (2006.01) Carella Byrne Brain Giffillan Cecchi StwartOlstein (52) U.S. Cl. ........................................ 424/94.6; 435/197 5 Becker Farm (57) ABSTRACT Roseland, NJ 07068 (US) The present invention provides for butyrylcholinesterase (BChE) attached to polyethylene glycol (PEG) to form a (21) Appl. No.: 12/309,909 complex having greatly increased mean residence time (MRT) in the system of an animal following injection there (22) PCT Filed: Aug. 2, 2007 into. Also disclosed are compositions of Such complexes, methods of preparing these complexes and method for using (86). PCT No.: PCT/US07/17279 these complexesp and compositionsp in the treatment and/or prevention of toxic effects of poisons, such as neurotoxins, to S371 (c)(1), which said animals, such as humans, have been, or may (2), (4) Date: Feb. 2, 2009 become, exposed. 1 2 3 4 Patent Application Publication Aug. 20, 2009 Sheet 1 of 2 US 2009/0208480 A1 Patent Application Publication Aug. 20, 2009 Sheet 2 of 2 US 2009/0208480 A1 SB 4 3 O O S. 2 5. ( 8 CD SS is'a J O N 9 lat1-al- Y O & S & 3 S & (u/n) ANOeguo US 2009/020848.0 A1 Aug. -

The Nervous System – Target Organ Into the Twenty-First Century
The Nervous System – Target Organ into the Twenty-First Century R. Douglas Hamm MD, CCFP, FRCPC (Occ Med), FCBOM President, Canadian Board of Occupational Medicine CONTENTS 1. WHY NEURONS MAKE GOOD TARGETS FOR OCCUPATIONAL TOXICANTS 2. FROM CLASSICAL PLUMBISM TO BEHAVIORAL TOXICOLOGY 3. TOXICOKINETICS, COMPARTMENTS, AND PBPK MODELS 4. THE DIVERSE TOXICODYNAMICS OF THE NERVOUS SYSTEM 1. Peripheral Neurons (neuronopathy, axonopathy, myelinopathy) 2. Synaptic Neurotransmission (cholinergic pathways) 3. Special Senses (visual, auditory, olfactory) 4. Movement Disorders (parkinsonism, ataxia, tremor) 5. Neuroaffective and Neurocognitive Effects 5. NOTEWORTHY OCCUPATIONAL NEUROTOXICANTS 1. “Heavy metals” (Pb, Hg, Tl) and other elements (Mn, As, Al, Sb, Te) 2. Organic Solvents (toluene, xylene, styrene, C2HCl3, C2Cl4, CH3CCl3, CS2) 3. Gases (HCN, CO, H2S, ethylene oxide) 4. Pesticides (organophosphates, carbamates, organochlorines, pyrethroids, neonicotinoids) 6. CLINICAL NEUROTOXICOLOGY 1. Identification of Occupational Neurotoxic Disorders 2. Biomarkers of Exposure and Effect 3. Clinical Investigations of Neurotoxicity 1. WHY NEURONS MAKE GOOD 2. FROM CLASSICAL PLUMBISM TO TARGETS FOR OCCUPATIONAL BEHAVIORAL TOXICOLOGY TOXICANTS Hippocrates (c. 460-370 BC) has been cited as Neuroanatomical structures have large surface the first ancient author to describe a case of areas and receptor populations, e.g., the occupational neurotoxicity but this has been surface area of the brain’s 100 billion neurons shown to be erroneous (Osler, 1907; Waldron, totals hundreds of square metres. 1973, 1978; Skrabanek, 1986; Vance, 2007). The earliest report appears to be that of Neurons have high rates of metabolism, e.g., Nicander of Colophon (2nd cent. BC) who the brain at 2% body mass consumes 20% of observed that in “psimuthion” i.e. -

The Targeted Pesticides As Acetylcholinesterase Inhibitors
Supplementary material The targeted pesticides as Acetylcholinesterase inhibitors: comprehensive cross-organism molecular modelling studies performed to anticipate the pharmacology of harmfulness to humans in vitro Milan Mladenović1,*, Biljana B. Arsić2,3, Nevena Stanković1, Nezrina Mihović1, Rino Ragno4,5, Andrew Regan6, Jelena S. Milićević7, Tatjana M. Trtić-Petrović7, Ružica Micić8 1 Kragujevac Center for Computational Biochemistry, Faculty of Science, University of Kragujevac, Radoja Domanovića 12, 34000 Kragujevac, P.O. Box 60, Republic of Serbia 2 Department of Mathematics, Faculty of Sciences and Mathematics, University of Niš, Višegradska 33, 18000 Niš, Republic of Serbia 3 Division of Pharmacy and Optometry, University of Manchester, Oxford Road, M13 9PT, Manchester, United Kingdom 4 Rome Center for Molecular Design, Department of Drug Chemistry and Technologies, Faculty of Pharmacy and Medicine, Sapienza Rome University, P.le A. Moro 5, 00185, Rome, Italy 5 Alchemical Dynamics srl 00125 Rome, Italy 6 School of Chemistry, University of Manchester, Oxford road, M13 9PL, Manchester, United Kingdom 7 Vinča Institute of Nuclear Sciences, University of Belgrade, PO Box 522, 11001 Belgrade, Republic of Serbia 8 Faculty of Sciences and Mathematics, University of Priština, Lole Ribara 29, 38220 Kosovska Mitrovica, Republic of Serbia * Correspondence: [email protected]; Tel.: +381-34-336-223, ext. 255 CONTENTS Figure S1. The sequence alignment between the Mus musculus and Homo sapiens AChE. Figure S2. Vina-based The SB alignment assessment of (a) co-crystallized mAChE inhibitor (PDB ID: 4A16), EC pink, ECRD yellow, RCRD green, ECCD black, RCCD red, and (b) co-crystallized hAChE inhibitor (PDB ID: 4BDT), EC pink, ECRD yellow, RCRD green, ECCD black, RCCD red. -

Interaction Profile for Mixture of Insecticides: Pyrethroids
44 5. References Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, et al. 2004. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J Toxicol Environ Health A 67(4):331-356. ATSDR. 1992. Public health assessment guidance manual. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. ATSDR. 2003a. Toxicological profile for pyrethrins and pyrethroids. Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/ToxProfiles/tp155.pdf. March 29, 2013. ATSDR. 2004a. Guidance manual for the assessment of joint toxic action of chemical mixtures. Atlanta, GA: Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/interactionprofiles/IP-ga/ipga.pdf. April 2, 2013. ATSDR. 2004b. Interaction profile for benzene, toluene, ethylbenzene, and xylenes (BTEX). Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/interactionprofiles/IP- btex/ip05.pdf. March 29, 2013. Belden JB, Lydy MJ. 2006. Joint toxicity of chlorpyrifos and esfenvalerate to fathead minnows and midge larvae. Environ Toxicol Chem 25(2):623-629. Bosgra S, van Eijkeren JC, van der Schans MJ, et al. 2009. Toxicodynamic analysis of the inhibition of isolated human acetylcholinesterase by combinations of methamidophos and methomyl in vitro. Toxicol Appl Pharmacol 236(1):1-8. Burr SA, Ray DE. 2004. Structure-activity and interaction effects of 14 different pyrethroids on voltage- gated chloride ion channels. Toxicol Sci 77(2):341-346. Cao Z, Shafer TJ, Crofton KM, et al. 2011. Additivity of pyrethroid actions on sodium influx in cerebrocortical neurons in primary culture. -

Toxicological Evaluation of Parathion and Azinphosmethyl in Freshwater Model Ecosystems
Y\v\&*io 6>\o C Toxicological evaluation of parathion and azinphosmethyl in freshwater model ecosystems NN08201.81B :R.J.Dorîland Toxicological evaluationo fparathio nan d azinphosmethyl infreshwate rmode l ecosystems Promotor: dr.J.H . Koeman,hoogleraa r ind e toxicologie. R.J. Dortland Toxicological evaluation of parathion and azinphosmethyl in freshwater model ecosystems Proefschrift terverkrijgin gva nd egraa dva n doctori nd elandbouwwetenschappen , op gezagva nd erecto rmagnificus , dr.H.C .va nde rPlas , hoogleraari nd eorganisch e scheikunde, inhe topenbaa rt everdedige n opvrijda g2 0jun i198 0 desnamiddag st evie ruu ri nd eaul a vand eLandbouwhogeschoo l teWageninge n Centre for Agricultural Publishing and Documentation Wageningen - 1980 Astract Dortland,R.J. ,1980 .Toxicologica l evaluationo fparathio nan dazinphosmethy li n freshwatermode l ecosystems.Agric .Res .Rep .(Versl .landbouwk .Onderz. )898 , ISBN9 022 0073 24 ,(viii )+ 112p. ,3 3figs ,4 2tables ,13 1refs ,appendices , Eng.an dDutc hsummaries . Also:Doctora l thesis,Wageningen . Astud ywa smad eo fth epossibl ehazard so f long-termexposur e offreshwate recosys temst olo w (<1 m gm~3 )concentration s oforganophosphoru s insecticides.Range-fin ding,acut ean dsub-acut e (3weeks )laborator y toxicity trialswer ecarrie d outwit h parathion,azinphosmethyl ,diazinon ,malathio nan dparathion-methyl .Th e rateso f dissipation ofthes ecompound s from thewate r phaseo faquari awit han dwithou tplant s andsediment swer estudied .Th emai ntes tspecie swer eDaphni amagna ,Asellu -

PDP Pesticide History Years Each Pesticide Was Reported from 1991 - 2020
USDA, AMS, S&T, MPD - Pesticide Data Program (PDP) 2 November 2020 PDP Pesticide History Years each pesticide was reported from 1991 - 2020 Pest Pesticide Name Code Years Pesticide was Reported 1,2,4-Triazole A68 2003 - 2007 1-Naphthol 382 1994 - 2000, 2003 - 2020 2,3,5-Trimethacarb A72 2020 2,4,5-T 312 2001 - 2016 2,4,5-TP AJE 2010 - 2013 2,4-D 026 1992 - 1998, 2002 - 2020 2,4-DB 317 1996 - 1997, 2003 - 2016, 2019 2,4-dimethyl aniline (2,4 DMA) AGQ 2007 - 2008 2,4-dimethylphenyl formamide (2,4-DMPF) AGR 2007 - 2012, 2016 - 2020 2,6-dichlorobenzamide 272 2010, 2017 2,6-DIPN AFZ 2016 - 2020 3,5-Dichloroaniline ABM 2003 3-Hydroxycarbofuran 512 1993 - 2020 3-ketocarbofuran 218 2001 - 2002 4,4-dibromobenzophenone AGS 2007 - 2008 4-Hydroxychlorothalonil ANM 2017 4-Hydroxydiphenylamine B19 1995 - 1997 5-Hydroxythiabendazole B28 1996 - 1997, 2003 - 2020 Abamectin 948 1994 - 1997, 1999, 2012 - 2020 Acephate 204 1991 - 2020 Acequinocyl AKS 2013 - 2015, 2020 Acetamiprid B80 2004 - 2020 Acetochlor 807 2001, 2003 - 2020 Acetochlor ethanesulfonic acid (ESA) ABN 2001 - 2013, 2017 Acetochlor oxanilic acid (OA) ABO 2001, 2003 - 2013 Acibenzolar S methyl B51 2003 - 2020 Acifluorfen 727 2003 - 2007, 2014 - 2017 Aclonifen D58 2017 - 2020 Acrinathrin A03 2012 - 2015, 2017 Afidopyropen F87 2020 Alachlor 227 1997 - 2020 Alachlor ethanesulfonic acid (ESA) ABP 2001 - 2013, 2017 Alachlor oxanilic acid (OA) ABQ 2001 - 2013 Aldicarb 167 1994 - 2020 Aldicarb sulfone 168 1994 - 2020 Aldicarb sulfoxide 169 1993 - 2020 Aldrin 001 1997 - 2020 Allethrin 002 1994, 1999 -
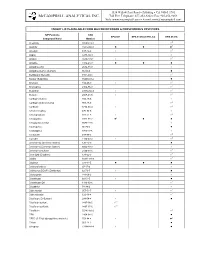
Compounds/Target Lists Detail
1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC. Toll Free Telephone: 877-252-9262 • Fax: 925-252-9269 Web: www.mccampbell.com • E-mail: [email protected] TARGET LISTS AVAILABLE FROM MAI FOR NITROGEN & PHOSPHOROUS PESTICIDES N/P Pesticide CAS EPA 507 EPA 8141A (CTR List) EPA 8141A Compound Name Number Acephate 30560-19-1 zZ Alachlor 15972-60-8 z z zZ Ametryn 834-12-8 z zZ Aspon 3244-90-4 z Atraton 1610-17-9 z zZ Atrazine 1912-24-9 z z z Azinphos ethyl 2642-71-9 z Azinphos methyl (Guthion) 86-50-0 z Benfluralin (Benefin) 1861-40-1 zZ Bolstar (Sulprofos) 35400-43-2 z Bromacil 314-40-9 z zZ Bromophos 2104-96-3 zZ Butachlor 23184-66-9 z zZ Butylate 2008-41-5 z zZ Carbophenothion 786-19-6 z Carbophenothion methyl 953-17-3 zZ Carboxin 5234-68-4 z zZ Chlorfenvinphos 470-90-6 z Chloropropham 101-21-3 z zZ Chlorpyrifos 2921-88-2 zZ z z Chlorpyrifos methyl 5598-13-0 z Coumaphos 56-72-4 z Crotoxyphos 7700-17-6 z Crufomate 299-86-5 zZ Cycloate 1134-23-2 z zZ Demeton-O (Demeton isomer) 126-75-0 z Demeton-S (Demeton isomer) 8065-48-3 z Demeton-S sulfone 2496-91-5 zZ Deet (Off) (Dephere) 134-62-3 zZ Dialifor 10311-84-9 zZ Diazinon 333-41-5 z z z Dichlorofenthion 97-17-6 z Dichlorvos (DDVP) (Dichlorfos) 62-73-7 z z Dicrotophos 141-66-2 z Dimethoate 60-51-5 z z Dimethoate OA 1113-02-6 zZ Dioxathion 78-34-2 z Diphenamid 957-51-7 z zZ Diphenylamine 122-39-4 zZ Disulfoton (Di-Syston) 298-04-4 z z Disulfoton sulfone 2497-06-5 zX1 Disulfoton sulfoxide 2497-07-6 zX1 Ethalflurin 55283-68-6 zZ EPN 2104-64-5 z EPTC (S-Ethyl dipropylthiocarbamate) 759-94-4 z zZ Ethion 563-12-2 z Ethoprop 13194-48-4 z z 1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC.