Organophosphate Poisoning : a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
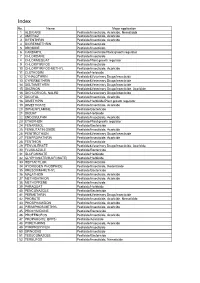
No. Name Major Application 1 ALDICARB Pesticide/Insecticide
Index No. Name Major application 1 ALDICARB Pesticide/Insecticide, Acaricide, Nematicide 2 AMITRAZ Pesticide/Insecticide, Acaricide 3 BIFENTHRIN Pesticide/Insecticide, Acaricide 4 BIORESMETHRIN Pesticide/Insecticide 5 BROMIDE Pesticide/Insecticide 6 CARBARYL Pesticide/Insecticide/Plant growth regulator 7 CHLORDANE Pesticide/Insecticide 8 CHLORMEQUAT Pesticide/Plant growth regulator 9 CHLORPYRIFOS Pesticide/Insecticide 10 CHLORPYRIFOS-METHYL Pesticide/Insecticide, Acaricide 11 CLETHODIM Pesticide/Herbicide 12 CYHALOTHRIN Pesticide&Veterinary Drugs/Insecticide 13 CYPERMETHRIN Pesticide&Veterinary Drugs/Insecticide 14 DELTAMETHRIN Pesticide&Veterinary Drugs/Insecticide 15 DIAZINON Pesticide&Veterinary Drugs/Insecticide, Acaricide 16 DICHLORVOS、NALED Pesticide&Veterinary Drugs/Insecticide 17 DICOFOL Pesticide/Insecticide, Acaricide 18 DIMETHIPIN Pesticide/Herbicide/Plant growth regulator 19 DIMETHOATE Pesticide/Insecticide, Acaricide 20 DIPHENYLAMINE Pesticide/Bactericide 21 DIQUAT Pesticide/Herbicide 22 ENDOSULFAN Pesticide/Insecticide, Acaricide 23 ETHEPHON Pesticide/Plant growth regulator 24 FENARIMOL Pesticide/Bactericide 25 FENBUTATIN OXIDE Pesticide/Insecticide, Acaricide 26 FENITROTHION Pesticide&Veterinary Drugs/Insecticide 27 FENPROPATHRIN Pesticide/Insecticide, Acaricide 28 FENTHION Pesticide/Insecticide 29 FENVALERATE Pesticide&Veterinary Drugs/Insecticide, Acaricide 30 FLUSILAZOLE Pesticide/Bactericide 31 GLUFOSINATE Pesticide/Herbicide 32 GLYPHOSATE(SULFOSATE) Pesticide/Herbicide 33 HEPTACHLOR Pesticide/Insecticide 34 HYDROGEN -

Propoxur United States Environmental Protection Agency
United States Prevention, Pesticides EPA738-R-97-009 Environmental Protection And Toxic Substances August 1997 Agency (7508W) Reregistration Eligibility Decision (RED) PROPOXUR UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide chemical case propoxur. The enclosed Reregistration Eligibility Decision (RED) contains the Agency's evaluation of the data base of this chemical, its conclusions of the potential human health and environmental risks of the current product uses, and its decisions and conditions under which these uses and products will be eligible for reregistration. The RED includes the data and labeling requirements for products for reregistration. It may also include requirements for additional data (generic) on the active ingredient to confirm the risk assessments. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED." This summary also refers to other enclosed documents which include further instructions. You must follow all instructions and submit complete and timely responses. The first set of required responses is due 90 days from the receipt of this letter. The second set of required responses is due 8 months from the date of receipt of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your products. If you have questions on the product specific data requirements or wish to meet with the Agency, please contact the Special Review and Reregistration Division representative Bonnie Adler (703) 308-8523. -

Carbamate Pesticides Aldicarb Aldicarb Sulfoxide Aldicarb Sulfone
Connecticut General Statutes Sec 19a-29a requires the Commissioner of Public Health to annually publish a list setting forth all analytes and matrices for which certification for testing is required. Connecticut ELCP Drinking Water Analytes Revised 05/31/2018 Microbiology Total Coliforms Fecal Coliforms/ E. Coli Carbamate Pesticides Legionella Aldicarb Cryptosporidium Aldicarb Sulfoxide Giardia Aldicarb Sulfone Carbaryl Physicals Carbofuran Turbidity 3-Hydroxycarbofuran pH Methomyl Conductivity Oxamyl (Vydate) Minerals Chlorinated Herbicides Alkalinity, as CaCO3 2,4-D Bromide Dalapon Chloride Dicamba Chlorine, free residual Dinoseb Chlorine, total residual Endothall Fluoride Picloram Hardness, Calcium as Pentachlorophenol CaCO3 Hardness, Total as CaCO3 Silica Chlorinated Pesticides/PCB's Sulfate Aldrin Chlordane (Technical) Nutrients Dieldrin Endrin Ammonia Heptachlor Nitrate Heptachlor Epoxide Nitrite Lindane (gamma-BHC) o-Phosphate Metolachlor Total Phosphorus Methoxychlor PCB's (individual aroclors) Note 1 PCB's (as decachlorobiphenyl) Note 1 Demands Toxaphene TOC Nitrogen-Phosphorus Compounds Alachlor Metals Atrazine Aluminum Butachlor Antimony Diquat Arsenic Glyphosate Barium Metribuzin Beryllium Paraquat Boron Propachlor Cadmium Simazine Calcium Chromium Copper SVOC's Iron Benzo(a)pyrene Lead bis-(2-ethylhexyl)phthalate Magnesium bis-(ethylhexyl)adipate Manganese Hexachlorobenzene Mercury Hexachlorocyclopentadiene Molybdenum Nickel Potassium Miscellaneous Organics Selenium Dibromochloropropane (DBCP) Silver Ethylene Dibromide (EDB) -

488 Subpart A—Organic Pesticide Chemicals Manufacturing
§ 455.11 40 CFR Ch. I (7–1–12 Edition) chemical products and be considered a this subpart are applicable to dis- ‘‘stand-alone’’ PFPR facility. charges resulting from the manufac- ture of the following organic active in- [43 FR 17776, Apr. 25, 1978, as amended at 50 FR 40701, Oct. 4, 1985; 51 FR 44911, Dec. 15, gredients: Aldrin, BHC, Captan, 1986; 58 FR 50689, Sept. 28, 1993; 61 FR 57548, Chlordane, DDD, DDE, DDT, Dichloran, Nov. 6, 1996] Dieldrin, Endosulfan, Endrin, Hepta- chlor, Lindane, Methoxychlor, Mirex, Subpart A—Organic Pesticide PCNB, Toxaphene, Trifluralin, Chemicals Manufacturing Azinphos Methyl, Demeton-O, Demeton-S, Diazinon, Disulfoton, Mal- Subcategory athion, Parathion Methyl, Parathion Ethyl, Aminocarb, Carbaryl, SOURCE: 43 FR 44846, Sept. 29, 1978, unless Methiocarb, Mexacarbate, Propoxur, otherwise noted. Barban, Chlorpropham, Diuron, Fenuron, Fenuron-TCA, Linuron, § 455.11 Compliance date for pretreatment standards for existing Monuron, Monuron-TCA, Neubron, sources (PSES). Propham, Swep, 2,4-D, Dicamba, Silvex, 2,4,5-T, Siduron, Perthane, and All discharges subject to Dicofol. pretreatment standards for existing (c) The intermediates used to manu- sources (PSES) in subparts A and B of facture the active ingredients and ac- this part must comply with the stand- tive ingredients used solely in experi- ards no later than September 28, 1993. mental pesticides are excluded from [61 FR 57549, Nov. 6, 1996] coverage in this subpart. Insecticidal pathogenic organisms such as Bacillus § 455.20 Applicability; description of thuringiensis, insect growth hormones, the organic pesticide chemicals plant extracts such as pyrethrins; sex manufacturing subcategory. attractants and botanicals such as Ro- (a) For the purpose of calculating and tenone are also excluded from BPT applying effluent limitations for COD, coverage in this subpart. -

Pesticides May Reduce Lettuce Yield Frank V
High-value crops such as strawberries, tain classes of insecticides on lettuce photo- usually clearly visible. Insecticides applied at broccoli, and iceberg lettuce often receive synthesis, transpiration, and productivity. normal rates and under the right environ- “preventive” or “insurance” pesticide treat- mental conditions may subtly damage a plant ments, which may result in weekly scheduled Insecticides but remain unobserved, because symptoms applications of insecticides. Many times such Insecticides in the various “classes,” such are not visible. treatments are unwarranted economically as chlorinated hydrocarbons (DDT, endrin, During the last few years, plant physiolo- and may reduce yields by detrimental effects and methoxychlor), organophosphates gists at University of California, Riverside, on the plants. Decreases in strawberry yields (guthion, parathion, and methyl-parathion), have developed the dual isotope porometer, due to preventive insecticide treatments in the carbamates (malathion and methomyl), and which provides accurate, simultaneous mea- absence of economically significant pest synthetic pyrethroids (fenvalerate and per- surements of a plant’s photosynthesis and populations have been reported. Research methrin), differ in their effects on plants. Ad- transpiration rates in the field. Entomologists supported by the California Iceberg Lettuce ditionally, the rates, number, and timing of have used the instrument to measure effects Research Advisory Board indicated that head applications may alter a compound’s effect -

Teratogenicity and Embryotoxicity of Organophosphorus Compounds in Animal Models - a Short Review
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2012, vol. 81(1), p. 16-26 ISSN 0372-7025 DOI: 10.31482/mmsl.2012.003 REVIEW ARTICLE TERATOGENICITY AND EMBRYOTOXICITY OF ORGANOPHOSPHORUS COMPOUNDS IN ANIMAL MODELS - A SHORT REVIEW Syed M Nurulain and M Shafiullah Department of Pharmacology and Therapeutic, Faculty of Medicine and Health Sciences, UAE University, AlAin, UAE. P.O.Box 17666, Alain, UAE Received 9 th December 2011. Revised 12 th February 2012. Published 9 th March 2012. Summary Organophosphorus compounds (OPCs) are a wide group of compounds both structurally and functionally. Each OPC has a unique toxicological profile. The exposure to this type of poison is not limited only to certain occupationally exposed people but also to children, women, pregnant women; all have chances to be exposed to this poison. During the recent past years it has been reported in many poison epidemiological studies and case reports that exposure of OPCs during pregnancy caused malformed fetuses, neural tube defect (NTD) and shortening of pregnancy. The literature for animal models reveals inconclusive evidence. The generalized view is that they are neither teratogenic nor embryotoxic. But it is not true. There is a lack of systematic study and scarcity of reports on the topic. The present study was undertaken to investigate the teratogenicity induced by organophosphorus compounds in different animal models by literature review. Literature was searched by Toxicology Data NetWork (TOXNET), Developmental and Reproductive Toxicology Database (DART), Toxicology Literature Online (TOXLINE), Hazardous Substances Data Bank (HSDB), Pubmed Central, Entrez-Pubmed, Science Direct, Directory Of Open Access Journal (DOAJ), Google Scholar and International Program on Chemical Safety (IPCS-INCHEM), Embase. -

Guide No. 1 – October 2020 2/12 the CONCEPT and IMPLEMENTATION of CPA GUIDANCE RESIDUE LEVELS
Cooperation Centre for Scientific Research Relative to Tobacco CORESTA GUIDE N° 1 The Concept and Implementation of CPA Guidance Residue Levels October 2020 Agro-Chemical Advisory Committee CORESTA TECHNICAL GUIDE N° 1 Title: The Concept and Implementation of CPA Guidance Residue Levels Status: Valid Note: This document will be periodically reviewed by CORESTA Document history: Date of review Information July 2003 Version 1 GRL for Pyrethrins () and Terbufos corrected. December 2003 CPA terminology corrected. June 2008 Version 2 – GRLs revised and residue definitions added Provisional GRL of 2.00 ppm for Cyfluthrin to replace previous June 2010 GRL of 0.50 ppm July 2013 Version 3 – GRLs revised October 2013 Note for Maleic Hydrazide revised Version 4 – GRLs revised + clarification that scope of GRLs July 2016 applies predominantly to the production of traditional cigarette tobaccos and GAP associated with their cultivation. June 2018 Fluopyram GRL of 5 ppm added to GRL list Version 5 – Nine new CPAs with GRL added to list. November 2019 Revision of GRLs for Chlorantraniliprole and Indoxacarb. Updated web links. October 2020 Version 6 – Flupyradifurone GRL of 21 ppm added to GRL list. CORESTA Guide No. 1 – October 2020 2/12 THE CONCEPT AND IMPLEMENTATION OF CPA GUIDANCE RESIDUE LEVELS Executive Summary • Guidance Residue Levels (GRLs) are in the remit of the Agro-Chemical Advisory Committee (ACAC) of CORESTA. Their development is a joint activity of all ACAC members, who represent the leaf production, processing and manufacturing sectors of the Tobacco Industry. The concept of GRLs and their implementation are described in this guide. • GRLs provide guidance to tobacco growers and assist with interpretation and evaluation of results from analyses of residues of Crop Protection Agents (CPAs*). -

Monitoring for Resistance to Organophosphorus and Pyrethroid Insecticides in Varroa Mite Populations
INSECTICIDE RESISTANCE AND RESISTANCE MANAGEMENT Monitoring for Resistance to Organophosphorus and Pyrethroid Insecticides in Varroa Mite Populations 1,2 3 1 4 LAMBERT H. B. KANGA, JOHN ADAMCZYK, KEITH MARSHALL, AND ROBERT COX J. Econ. Entomol. 103(5): 1797Ð1802 (2010); DOI: 10.1603/EC10064 ABSTRACT The occurrence of resistance in Varroa mite populations is a serious threat to the beekeeping industry and to crops that rely on the honey bee for pollination. Integrated pest management strategies for control of this pest include the judicious use of insecticides. To monitor Þeld populations of Varroa mite for insecticide resistance, a glass vial bioassay procedure was developed to use in the development of a resistance management strategy. Diagnostic concen- trations needed to separate susceptible genotypes from resistant individuals were determined for cypermethrin (0.1 g per vial), ßuvalinate (5.0 g per vial), malathion (0.01 g per vial), coumaphos (10.0 g per vial), diazinon (5.0 g per vial), methomyl (0.5 g per vial), propoxur (0.1 g per vial), and endosulfan (2.5 g per vial). Resistance to organophosphorus insecticides (malathion, coumaphos) and pyrethroids (cypermetrhrin, ßuvalinate) was widespread in both La Media Ranch, TX, and Wewahitchka, FL, from 2007 to 2009. There was no resistance to endosulfan, diazinon, methomyl, and propoxur in Þeld populations of Varroa mite in the two locations where resistance was monitored. The seasonal patterns of resistance in Wewahitchka were different from those of La Media Ranch. In the former location, the frequency of resistance to all insecticides tested decreased signiÞcantly from 2007 to 2009, whereas it increased in the latter location. -

2018 Treated Water Undetected Chemical Contaminant List
2018 Treated Water Undetected Chemical Contaminant List ESTROGENS AND OTHER HORMONES Diethylstilbestrol (DES) Estrone 17alpha-Estradiol 17alpha-Ethynal estradiol 17beta-Estradiol Progesterone Estriol cis-Testosterone trans-Testosterone INORGANIC CHEMICALS Antimony Niobium Arsenic Osmium Beryllium Palladium Cadmium Platinum Cerium Praseodymium Cesium Rhenium Cobalt Rhodium Cyanide Ruthenium Dysprosium Samarium Erbium Selenium Europium Silver Gadolinium Tantalum Gallium Tellurium Germanium Thallium Gold Thorium Hafnium Thulium Holmium Tin Iridium Titanium Lanthanum Tungsten Lead Uranium Lutetium Vanadium Mercury Ytterbium Molybdenum Zinc Neodymium Zirconium Nickel NITROSAMINES N-Nitropyrrolidine (NPYR) N-Nitrosomorpholine (NMOR) N-Nitrosodi-N-butylamine (NDBA) N-Nitrosodiphenylamine (NDPhA) N-Nitrosodiethylamine (NDEA) N-Nitrosodi-N-propylamine (NDPA) N-Nitrosodimethylamine (NDMA) N-Nitrosomethylethylamine (NMEA) N-Nitrosopiperidine (NPIP) 1 ORGANIC CHEMICALS Acenaphthene Butylbenzylphthalate Acenaphthylene Butyraldehyde (Butanal) Acetaldehyde Carbaryl Acetochlor Carbofuran Acetone Carbon disulfide Acrylamide Carbophenothion Acrylonitrile Carbon tetrachloride Alachlor Carboxin Aldicarb (Temik) Chlordane Aldicarb sulfone Chlordane, alpha Aldicarb sulfoxide Chlordane, gamma Aldrin Chlorfenvinphos Allyl chloride Chloroacetonitrile Tert-Amyl Methyl ether Chlorobenzene Ametryn Chlorobenzilate Anilizine 2-Chlorobiphenyl Anthracene 1-Chlorobutane Aspon Chloroethane Atraton Chloromethane Atrazine Chloroneb Azinphos-ethyl Chloroprene Azinphos-methyl -

2002 NRP Section 6, Tables 6.1 Through
Table 6.1 Scoring Table for Pesticides 2002 FSIS NRP, Domestic Monitoring Plan } +1 0.05] COMPOUND/COMPOUND CLASS * ) (EPA) (EPA) (EPA) (EPA) (EPA) (FSIS) (FSIS) PSI (P) TOX.(T) L-1 HIST. VIOL. BIOCON. (B) {[( (2*R+P+B)/4]*T} REG. CON. (R) * ENDO. DISRUP. LACK INFO. (L) LACK INFO. {[ Benzimidazole Pesticides in FSIS Benzimidazole MRM (5- 131434312.1 hydroxythiabendazole, benomyl (as carbendazim), thiabendazole) Carbamates in FSIS Carbamate MRM (aldicarb, aldicarb sulfoxide, NA44234416.1 aldicarb sulfone, carbaryl, carbofuran, carbofuran 3-hydroxy) Carbamates NOT in FSIS Carbamate MRM (carbaryl 5,6-dihydroxy, chlorpropham, propham, thiobencarb, 4-chlorobenzylmethylsulfone,4- NT 4 1 3 NV 4 4 13.8 chlorobenzylmethylsulfone sulfoxide) CHC's and COP's in FSIS CHC/COP MRM (HCB, alpha-BHC, lindane, heptachlor, dieldrin, aldrin, endrin, ronnel, linuron, oxychlordane, chlorpyrifos, nonachlor, heptachlor epoxide A, heptachlor epoxide B, endosulfan I, endosulfan I sulfate, endosulfan II, trans- chlordane, cis-chlordane, chlorfenvinphos, p,p'-DDE, p, p'-TDE, o,p'- 3444NV4116.0 DDT, p,p'-DDT, carbophenothion, captan, tetrachlorvinphos [stirofos], kepone, mirex, methoxychlor, phosalone, coumaphos-O, coumaphos-S, toxaphene, famphur, PCB 1242, PCB 1248, PCB 1254, PCB 1260, dicofol*, PBBs*, polybrominated diphenyl ethers*, deltamethrin*) (*identification only) COP's and OP's NOT in FSIS CHC/COP MRM (azinphos-methyl, azinphos-methyl oxon, chlorpyrifos, coumaphos, coumaphos oxon, diazinon, diazinon oxon, diazinon met G-27550, dichlorvos, dimethoate, dimethoate -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Pesticide Analytical Manual Volume I 10/97 Revisions
Pesticide Analytical Manual Volume I 10/97 Revisions The following pages contain corrections or changes for PAM I. Print these pages and use them to replace the same current pages in the current PAM I 3rd edition (published 1/94, revised 9/96). Each set of two pages is intended to appear on two sides of the same paper, but Acrobat Reader does not offer a feature that facilitates printing on both sides of the page. It may be necessary to print one page at a time and turn the paper over to print the second page on the reverse side. Material Transmitted PAM I Page Page of this File 1. Title Page i, ii (not numbered on page) 2, 3 2. Introduction v, vi 4, 5 3. Chapter 1 Table of Contents 100-1 to 100-4 6-9 4. Section 105 105-1 to 105-4 10-13 5. Chapter 2 Table of Contents 200-1 to 200-4 14-17 6. Chapter 3 Table of Contents 300-1 to 300-4 18-21 7. Pages 302-3 and 302-4 302-3, 302-4 22-23 8. Pages 302-23 and 302-24 302-23, 302-24 24-25 9. Pages 302-27 and 302-28 302-27, 302-28 26-27 10. Pages 302-33 and 302-34 302-33, 302-34 28-29 11. Pages 302-51 and 302-52 302-51, 302-52 30-31 12. Pages 302-57 and 302-58 302-57, 302-58 32-33 13. Pages 302-63 to 302-70 302-63 to 302-70 34-41 14.