Microtubules and the Endoplasmic Reticulum Are Highly Interdependent Structures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physical and Chemical Basis of Cytoplasmic Streaming
Annual Reviews www.annualreviews.org/aronline .4n~t Rev. Plant Physiol 1981. 32:205-36 Copyright© 1981by AnnualReviews In~ All rights reserved PHYSICAL AND CHEMICAL BASIS OF CYTOPLASMIC ~7710 STREAMING Nobur6 Kamiya Department of Cell Biology, National Institute for Basic Biology, Okazaki, 444 Japan CONTENTS INTRODUCTION........................................................................................................ 206 SHUTI’LE STREAMINGIN THE MYXOMYCETEPLASMODIUM ................ 207 General...................................................................................................................... 207 ContractileProperties of the PlasmodialStrand ...................................................... 208 Activationcaused by stretching .................................................................................. 208 Activationcaused by loading .................................................................................... 209 Synchronizationof local ,hythms .............................................................................. 209 ContractileProteins .................................................................................................. 210 Plasmodiumactomyosin .......................................................................................... 210 Plusmodiummyosin ................................................................................................ 210 Plusmodiumactin.................................................................................................... 211 -

Overview of the Cytoskeleton from an Evolutionary Perspective
Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press Overview of the Cytoskeleton from an Evolutionary Perspective Thomas D. Pollard1 and Robert D. Goldman2 1Departments of Molecular Cellular and Developmental Biology, Molecular Biophysics and Biochemistry, and Cell Biology ,Yale University, New Haven, Connecticut 06520-8103 2Department of Cell and Molecular Biology, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611 Correspondence: [email protected] SUMMARY Organisms in the three domains of life depend on protein polymers to form a cytoskeleton that helps to establish their shapes, maintain their mechanical integrity, divide, and, in many cases, move. Eukaryotes have the most complex cytoskeletons, comprising three cytoskeletal poly- mers—actin filaments, intermediate filaments, and microtubules—acted on by three families of motor proteins (myosin, kinesin, and dynein). Prokaryotes have polymers of proteins ho- mologous to actin and tubulin but no motors, and a few bacteria have a protein related to intermediate filament proteins. Outline 1 Introduction—Overview of cellular 4 Overview of the evolution of the cytoskeleton functions 5 Conclusion 2 Structures of the cytoskeletal polymers References 3 Assembly of cytoskeletal polymers Editors: Thomas D. Pollard and Robert D. Goldman Additional Perspectives on The Cytoskeleton available at www.cshperspectives.org Copyright # 2018 Cold Spring Harbor Laboratory Press; all rights reserved; doi: 10.1101/cshperspect.a030288 Cite this article as Cold Spring Harb Perspect Biol 2018;10:a030288 1 Downloaded from http://cshperspectives.cshlp.org/ on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press T.D. Pollard and R.D. Goldman 1 INTRODUCTION—OVERVIEW OF CELLULAR contrast, intermediate filaments do not serve as tracks for FUNCTIONS molecular motors (reviewed by Herrmann and Aebi 2016) but, rather, are transported by these motors. -

The Mechanics of Motility in Dissociated Cytoplasm
THE MECHANICS OF MOTILITY IN DISSOCIATED CYTOPLASM MICAH DEMBO Theoretical Biophysics, Theoretical Division, Los Alamos National Laboratory, Group T-10, Mail Stop K710, Los Alamos, New Mexico 87545 ABSTRACT We stimulate the dynamical behavior of dissociated cytoplasm using the Reactive Flow Model (Dembo, M., and F. Harlow, 1986, Biophys. J., 50:109-121). We find that for the most part the predicted dynamical behavior of the cytoplasm is governed by three nondimensional numbers. Several other nondimensional parameters, the initial conditions, and boundary conditions are found to have lesser effects. Of the three major nondimensional parameters, one (D#) controls the percentage of ectoplasm, the second (CO) controls the sharpness of the endoplasm-ectoplasm boundary, and the third (R#) controls the topological complexity of the endoplasm-ectoplasm distribution. If R# is very small, then the cytoplasm contracts into a single uniform mass, and there is no bulk streaming. If R# is very large, then the cytoplasmic mass breaks up into a number of clumps scattered throughout the available volume. Between these clumps the solution undergoes turbulent or chaotic patterns of streaming. Intermediate values of R# can be found such that the mass of cytoplasm remains connected and yet undergoes coherent modes of motility similar to flares (Taylor, D.L., J.S. Condeelis, P.L. Moore, and R.D. Allen, 1973, J. Cell Biol., 59:378-394) and rosettes (Kuroda, K., 1979, Cell Motility: Molecules and Organization, 347-362). INTRODUCTION (Dembo et al., 1986). Here we will consider two experi- in which chemical reaction is an essential The reactive flow model is a putative description of motile mental systems fluid part of the dynamics. -

Cell & Molecular Biology
BSC ZO- 102 B. Sc. I YEAR CELL & MOLECULAR BIOLOGY DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY BSCZO-102 Cell and Molecular Biology DEPARTMENT OF ZOOLOGY SCHOOL OF SCIENCES UTTARAKHAND OPEN UNIVERSITY Phone No. 05946-261122, 261123 Toll free No. 18001804025 Fax No. 05946-264232, E. mail [email protected] htpp://uou.ac.in Board of Studies and Programme Coordinator Board of Studies Prof. B.D.Joshi Prof. H.C.S.Bisht Retd.Prof. Department of Zoology Department of Zoology DSB Campus, Kumaun University, Gurukul Kangri, University Nainital Haridwar Prof. H.C.Tiwari Dr.N.N.Pandey Retd. Prof. & Principal Senior Scientist, Department of Zoology, Directorate of Coldwater Fisheries MB Govt.PG College (ICAR) Haldwani Nainital. Bhimtal (Nainital). Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Programme Coordinator Dr. Shyam S.Kunjwal Department of Zoology School of Sciences, Uttarakhand Open University Haldwani, Nainital Unit writing and Editing Editor Writer Dr.(Ms) Meenu Vats Dr.Mamtesh Kumari , Professor & Head Associate. Professor Department of Zoology, Department of Zoology DAV College,Sector-10 Govt. PG College Chandigarh-160011 Uttarkashi (Uttarakhand) Dr.Sunil Bhandari Asstt. Professor. Department of Zoology BGR Campus Pauri, HNB (Central University) Garhwal. Course Title and Code : Cell and Molecular Biology (BSCZO 102) ISBN : 978-93-85740-54-1 Copyright : Uttarakhand Open University Edition : 2017 Published By : Uttarakhand Open University, Haldwani, Nainital- 263139 Contents Course 1: Cell and Molecular Biology Course code: BSCZO102 Credit: 3 Unit Block and Unit title Page number Number Block 1 Cell Biology or Cytology 1-128 1 Cell Type : History and origin. -

Acanthamoeba Is an Evolutionary Ancestor of Macrophages: a Myth Or Reality? Ruqaiyyah Siddiqui Aga Khan University
eCommons@AKU Department of Biological & Biomedical Sciences Medical College, Pakistan February 2012 Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Ruqaiyyah Siddiqui Aga Khan University Naveed Ahmed Khan Aga Khan University Follow this and additional works at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs Part of the Biochemistry Commons Recommended Citation Siddiqui, R., Khan, N. (2012). Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality?. Experimental Parasitology, 130(2), 95-97. Available at: http://ecommons.aku.edu/pakistan_fhs_mc_bbs/16 Experimental Parasitology 130 (2012) 95–97 Contents lists available at SciVerse ScienceDirect Experimental Parasitology journal homepage: www.elsevier.com/locate/yexpr Minireview Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? ⇑ Ruqaiyyah Siddiqui a, Naveed Ahmed Khan a,b, a Aga Khan University, Stadium Road, Karachi, Pakistan b School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, England, UK article info abstract Article history: Given the remarkable similarities in cellular structure (morphological and ultra-structural features), Received 26 October 2011 molecular motility, biochemical physiology, ability to capture prey by phagocytosis and interactions with Received in revised form 17 November 2011 microbial pathogens, here we pose the question whether Acanthamoeba and macrophages are evolution- Accepted 19 November 2011 ary related. This is discussed in the light of evolution and functional aspects such as the astonishing Available online 28 November 2011 resemblance of many bacteria to infect and multiply inside human macrophages and amoebae in analo- gous ways. Further debate and studies will determine if Acanthamoeba is an evolutionary ancestor of Keywords: macrophages. Is this a myth or reality? Acanthamoeba Ó 2011 Elsevier Inc. -
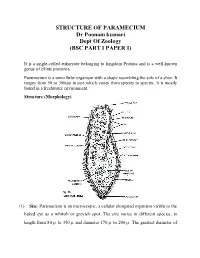
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

EE Just's "Independent Irritability"
ESSAY Molecular Reproduction & Development 76:966–974 (2009) E.E. Just’s ‘‘Independent Irritability’’ Revisited: The Activated Egg as Excitable Soft Matter STUART A. NEWMAN* Department of Cell Biology and Anatomy, New York Medical College, Valhalla, New York SUMMARY Ernest Everett Just’s experimental work on post-fertilization events in invertebrate eggs led him to posit a dynamic and directive role for the zygotic ‘‘ectoplasm’’ (cortical Just was correct in his estimation cytoplasm), in subsequent development. His perspective was neglected during the of the ‘‘informational’’ role of the years that followed his early death not only because of his well-documented margina- ectoplasm’s dynamics. lization as an African-American in U.S. science, but because his ideas were at odds with the growing gene-centrism of developmental biology in the latter half of the 20th century. This essay reviews experimental work that shows that the egg cortex in many animal groups is a chemically and mechanically active medium that sustains both spatiotemporal calcium ion transients and periodic deformations in the time leading up * Corresponding author: to cleavage. These wave phenomena are seen to play regulatory roles in germ plasm Department of Cell Biology and localization and gene expression, and influence the reliability and success of devel- Anatomy opmental outcomes. Just resisted vitalistic explanations for the active processes he New York Medical College Basic Science Building observed and inferred regarding the egg cortical cytoplasm, but recognized that the Valhalla, NY 10595. physics and chemistry of his time were inadequate to account for these phenomena E-mail: [email protected] and anticipated that expansions of these fields would be necessary to explain them. -

Cytoskeleton and Cell Motility
Cytoskeleton and Cell Motility 1. (28 pts) Amoeba proteus, a single-celled eukaryote, moves by means of psudopods attaching to and detaching from the substratum. Locomotion seems to be correlated with the forward flow of fluid cytoplasm (endoplasm) into an advancing pseudopod through a surrounding, gel-like ectoplasmic tube. The ectoplasm forms at the pseudopodial tip in a region called the Fountain Zone. As the amoeba advances the ectoplasmic tube “liquifies” at the posterior end to form endoplasm. These features are illustrated in the figure below. Both locomotion and cytoplasmic streaming are inhibited by cytochalasin B. Consider everything you’ve learned so far and answer all the following questions. A. (4 pts) When amoeba undergoes cell division, it stops streaming and rounds up into a spherical cell. Describe how this change in shape and behavior comes about and why it might be a necessary precondition for division. B. (6 pts) Briefly describe how cytoplasmic streaming is most likely organized and generated at the cellular and molecular levels. C. (5 pts) Briefly describe an additional experiment or observation that would test your hypothesis and indicate clearly what the results would show. CCM - 1 Cytoskeleton and Cell Motility D. (8 pts) Describe clearly, with the aid of a well-labeled diagram, how streaming within a pseudopod could result in movement of the amoeba across the substratum. E. (5 pts) Describe how your streaming mechanism might be regulated such that the amoeba might change its streaming pattern to form phagocytic pseudopods around a ciliate it had touched. Now evaluate some past answers to these questions, in light of your own essays. -

Structure and Development of the Egg of the Glossiphoniid Leech Theromyzon Rude: Characterization of Developmental Stages and Structure of the Early Uncleaved Egg
Development 100, 211-225 (1987) 211 Printed in Great Britain © The Company of Biologists Limited 1987 Structure and development of the egg of the glossiphoniid leech Theromyzon rude: characterization of developmental stages and structure of the early uncleaved egg JUAN FERNANDEZ, NANCY OLEA and CECILIA MATTE Departamento de Biologia, Facultad de Ciendas, Untversidad de Chile, Casilla 653, Santiago, Chile Summary Some aspects of the reproductive biology of the meridional bands, during stage le, lead to accumu- glossiphoniid leech, Theromyzon rude, under labora- lation of ooplasm at both egg poles. In this manner, tory conditions, and the staging and structure of its the teloplasm or pole plasm forms. Completion of the uncleaved egg were studied. Sexually mature animals first cleavage furrow, by the end of stage If, is form breeding communities and fertilization occurs in preceded by dorsoventral flattening of the egg and the ovLsacs, presumably around the time of egg rearrangement of its teloplasm and perinuclear laying. Opposition may be postponed for hours or plasm. Structure of the early uncleaved egg has been days, but the eggs in the ovisacs remain blocked at studied with transmission and scanning electron mi- first meiotic metaphase. Development of the croscopy of intact or permeabilized preparations. The uncleaved egg, from the time of oviposit ion to com- plasmalemma forms numerous long and some short pletion of the first cleavage division, has been sub- microvilli evenly distributed across the egg surface. divided into six stages. At 20 °C, the six developmental The ectoplasm includes many vesicles, mitochondria, stages take 5-6 h. Characterization of' the stages is granules and an elaborate network of filament based on observations of both live and fixed/cleared bundles. -

The Mechanism of Cytoplasmic Streaming in Characean Algal Cells: Sliding of Endoplasmic Reticulum Along Actin Filaments Bechara Kachar* and Thomas S
The Mechanism of Cytoplasmic Streaming in Characean Algal Cells: Sliding of Endoplasmic Reticulum along Actin Filaments Bechara Kachar* and Thomas S. Reese Laboratory of Neum-otolaryngology*and Laboratory of Neumbiology, National Institute of Neurological and Communicative Disorders and Stroke, National Institutes of Health, Bethesda, Maryland 20205 Abstract. Electron microscopy of directly frozen giant is dissociated in a buffer containing ATP. The shear cells of characean algae shows a continuous, tridimen- forces produced at the interface with the dissociated sional network of anastomosing tubes and cisternae of actin cables move large aggregates of endoplasmic rough endoplasmic reticulum which pervade the reticulum and other organelles. The combination of streaming region of their cytoplasm. Portions of this fast-freezing electron microscopy and video micros- endoplasmic reticulum contact the parallel bundles of copy of living cells and dissociated cytoplasm demon- actin filaments at the interface with the stationary cor- strates that the cytoplasmic streaming depends on en- tical cytoplasm. Mitochondria, glycosomes, and other doplasmic reticulum membranes sliding along the small cytoplasmic organelles enmeshed in the endo- stationary actin cables. Thus, the continuous network plasmic reticulum network display Brownian motion of endoplasmic reticulum provides a means of exerting while streaming. The binding and sliding of endoplas- motive forces on cytoplasm deep inside the cell distant mic reticulum membranes along actin cables can also from the cortical actin cables where the motive force be directly visualized after the cytoplasm of these cells is generated. uE coordinated movement of intraceUular elements, Several reports (2, 3, 5, 14, 16, 19) have dealt with the ques- the cytoplasmic streaming, in giant characean algae tion whether the active movement of putative organelles, T cells was discovered by Corti in 1774 (for review see using the translocator molecules on their surfaces to move reference 14). -

Composition of Cytoplasm
Cytoplasm Definition Cytoplasm is the semi-fluid substance of a cell that is present within the cellular membrane and surrounds the nuclear membrane. It is sometimes described as the nonnuclear content of the protoplasm. All the cellular contents in a prokaryote organisms are contained within cell's cytoplasm. In eukaryote organisms, the nucleus of the cell is separated from the cytoplasm. Cytoplasm is a thick and semi-transparent fluid. The cytoplasm was discovered in the year 1835 by Robert Brown and other scientists. The cytoplasm is made of 70% - 90% water and is colorless usually. Most of the cellular activities occurs in the cytoplasm. Metabolic pathways like glycolysis and cellular processes like cell division take place in the cytoplasm. The outer clear and glassy layer of the cytoplasm is called the ectoplasm or the cell cortex and the inner granular mass is called the endoplasm. In plants cells, a process known as cytoplasmic streaming takes place where there is movements of the cytoplasm around the vacuoles. General Characteristics of Cytoplasm: • Cytoplasm is the fluid substance that fills the space between the cell membrane and the cellular organelles. • Cytoplasm shows differential staining properties, the areas stained with the basic dyes are the basophilic areas of the cytoplasm and is termed as ergatoplasm for this material. • It is heterogenous mixture of opaque granules and organic compounds which gives it its colloidal nature. • The peripheral zone of cytoplasm is thick and jelly-like substance, known as the plasmogel. The surrounding area of the nuclear zone is thin and liquefied in nature and is known as the plasmosol. -

I-Viii Cshperspect-ERT-FM 1..8
This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. The Endoplasmic Reticulum A subject collection from Cold Spring Harbor Perspectives in Biology © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. OTHER SUBJECT COLLECTIONS FROM COLD SPRING HARBOR PERSPECTIVES IN BIOLOGY Wnt Signaling Protein Synthesis and Translational Control The Synapse Extracellular Matrix Biology Protein Homeostasis Calcium Signaling The Golgi Germ Cells The Mammary Gland as an Experimental Model The Biology of Lipids: Trafficking, Regulation, and Function Auxin Signaling: From Synthesis to Systems Biology The Nucleus Neuronal Guidance: The Biology of Brain Wiring Cell Biology of Bacteria Cell–Cell Junctions Generation and Interpretation of Morphogen Gradients Immunoreceptor Signaling NF-kB: A Network Hub Controlling Immunity, Inflammation, and Cancer Symmetry Breaking in Biology The Origins of Life The p53 Family SUBJECT COLLECTIONS FROM COLD SPRING HARBOR PERSPECTIVES IN MEDICINE Addiction Parkinson’s Disease Type 1 Diabetes Angiogenesis: Biology and Pathology HIV: From Biology to Prevention and Treatment The Biology of Alzheimer Disease © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. The Endoplasmic Reticulum A subject collection from Cold Spring Harbor Perspectives in Biology EDITED BY Susan Ferro-Novick Tom A. Rapoport University of California San Diego Harvard Medical School Randy Schekman University of California at Berkeley COLD SPRING HARBOR LABORATORY PRESS Cold Spring Harbor, New York † www.cshlpress.org © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum.