A Comparison Between Triplet and Doublet Chemotherapy in Improving
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
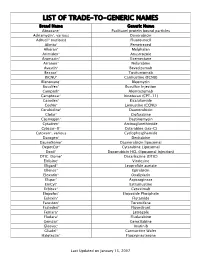
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

A Phase II Study of Paclitaxel and Capecitabine As a First-Line Combination Chemotherapy for Advanced Gastric Cancer
British Journal of Cancer (2008) 98, 316 – 322 & 2008 Cancer Research UK All rights reserved 0007 – 0920/08 $30.00 www.bjcancer.com A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer Clinical Studies HJ Kang1, HM Chang1, TW Kim1, M-H Ryu1, H-J Sohn1, JH Yook2,STOh2, BS Kim2, J-S Lee1 and Y-K Kang*,1 1 2 Division of Oncology, Department of Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea; Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea Paclitaxel and capecitabine, which have distinct mechanisms of action and toxicity profiles, have each shown high activity as single agents in gastric cancer. Synergistic interaction between these two drugs was suggested by taxane-induced upregulation of thymidine phosphorylase. We, therefore, evaluated the antitumour activity and toxicities of paclitaxel and capecitabine as first-line therapy in patients with advanced gastric cancer (AGC). Patients with histologically confirmed unresectable or metastatic AGC were treated À2 À2 with capecitabine 825 mg m p.o. twice daily on days 1–14 and paclitaxel 175 mg m i.v. on day 1 every 3 weeks until disease progression or unacceptable toxicities. Between June 2002 and May 2004, 45 patients, of median age 57 years (range ¼ 38–73 years), were treated with the combination of capecitabine and paclitaxel. After a median 6 cycles (range ¼ 1–9 cycles) of chemotherapy, 43 were evaluable for toxicity and response. A total of 2 patients showed complete response and 20 showed partial response making the overall response rate 48.9% (95% CI ¼ 30.3–63.5%). -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

1. Recommendations of the Ndac (Oncology and Hematology) Held on 10.12.2011
1. RECOMMENDATIONS OF THE NDAC (ONCOLOGY AND HEMATOLOGY) HELD ON 10.12.2011:- The NDAC (Oncology and Hematology) deliberated the proposals on 10.12.2011 and recommended the following:- AGENDA NAME OF DRUG RECOMMENDATIONS NO. Global Clinical Trials Recommended for giving permission for clinical trial subject to condition that trasuzumab naive patient arm should be excluded from the study, as patients cannot be left untreated of trastuzumab therapy / or 1 Afatinib treated with only investigational drug. Patients aged 18 to 65 years should be included in the study. Recommended for giving permission for clinical trial subject to the following conditions:- ICD is very extensive and too technical for the 2 Netupitant/ patient to understand. It should be simplified. Palonosetron Definite statistical tests for the comparison of primary and secondary endpoints should be incorporated in the protocol. Recommended for giving permission for clinical trial subject to the following conditions:- Periodic ophthalmic examination should be performed at every visit as the drug is reported to have ophthalmological side effects in 53 % cases in 3 Crizotinib phase 2 study. Patients aged 18 to 65 years should be included in the protocol. Method for causality assessment by the investigator should be included in the protocol. New Drugs Approved with the condition that structured post marketing trial (Phase 4) should be conducted in 4. Indian population. Report of post marketing trials Crizotinib ongoing in other countries when completed should be submitted. 5. Abiraterone Approved with condition of conducting Post Acetate Marketing trial (Phase IV) in Indian population to monitor the adverse effects. Report of post marketing trials ongoing in other countries when completed should be submitted. -

Covalently-Linked Complexes and Methods for Enhanced Cytotoxicity and Imaging
Europaisches Patentamt 19 European Patent Office Office europeen des brevets © Publication number: 0 359 347 B1 12 EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification © int. ci.5: A61K 47/42, A61K 49/02, 23.12.92 Bulletin 92/52 A61K 43/00 © Application number : 89250014.1 © Date of filing : 14.08.89 @) Covalently-linked complexes and methods for enhanced cytotoxicity and imaging. © Priority : 15.08.88 US 232337 © Proprietor : NEORX CORPORATION 410 West Harrison Street Seattle Washington 98119 (US) @ Date of publication of application 21.03.90 Bulletin 90/12 © Inventor : Anderson, David C. 2415 Thorndyke Avenue No.301 © Publication of the grant of the patent : Seattle Washington 98199 (US) 23.12.92 Bulletin 92/52 Inventor : Morgan, A. Charles 803 Driftwood Place Edmonds Washington 98020 (US) @ Designated Contracting States : Inventor : Abrams, Paul G. AT BE CH DE ES FR GB GR IT LI LU NL SE 2125 First Avenue No. 1602 Seattle Washington 98121 (US) Inventor : Nichols, Everett J. © References cited : 12525 17th Avenue N.E. EP-A- 0 282 057 Seattle Washington 98125 (US) CHEMICAL ABSTRACTS, vol. 108, no. 7, 1987, Inventor : Fritzberg, Alan R. abstract no. 48913a, Columbus, Ohio, US; J.M. 16703 74th Place West BOGGS et al.: Edmonds Washington 98020 (US) "Antigen-targetedliposome-encapsulated methotrexate specifically kills lymphocytes sensitized to the nonapeptide of myelin basic © Representative : Wablat, Wolfgang, Dr. Dr. protein ", & J.NEUROIMMUNOL, 17(1), 35-48 Potsdamer Chaussee 48 W-1000 Berlin 38 (DE) CO h- CO If) CO Note : Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0216288 A1 Chang (43) Pub
US 20060216288A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0216288 A1 Chang (43) Pub. Date: Sep. 28, 2006 (54) COMBINATIONS FOR THE TREATMENT OF Publication Classification CANCER (51) Int. Cl. (75) Inventor: David Chang, Calabasas, CA (US) A 6LX 39/395 (2006.01) A6II 3/55 (2006.01) Correspondence Address: A6II 3 L/4545 (2006.01) SESS is 2-C A61K 31/4439 (2006.01) ONE AMGEN CENTERY DRIVE A6II 3/44 (2006.O1 ) THOUSAND OAKS, CA 91320-1799 (US) (52) U.S. Cl. ................... 424/143.1: 514/352: 514/210.2: (73) Assignee: Amgen Inc., Thousand Oaks, CA 514/318: 514/340; 514/217.04 (21) Appl. No.: 11/386,271 (22) Filed: Mar. 21, 2006 (57) ABSTRACT Related U.S. Application Data This invention is in the field of pharmaceutical agents and (60) Provisional application No. 60/664,381, filed on Mar. specifically relates to compounds, compositions, uses and 22, 2005. methods for treating cancer. Patent Application Publication Sep. 28, 2006 Sheet 1 of 5 US 2006/0216288A1 Figure 1 -- Vehicle X Compound B, 10 mpk 1800 -- Antibody A, 20 ug 1600 Compound B, 10 mpk+ 1400 Antibody A, 20 ug 1200 1000 800 600 p = 0.0003 1/10 1110 1/10 p < 0.0001 v v v v v v V 174. 22 27 32 37 42 47 Time (days) V Antibody A injection Patent Application Publication Sep. 28, 2006 Sheet 2 of 5 US 2006/0216288A1 Figure 2 -- Vehicle 1800 X Compound B, 75 mpk 1600 th- Antibody A, 500 u 1400 dy 9 1200 Compound B, 75 mpk+ Antibody A, 500 ug 1000 800 600 V Antibodyy A, ipp injectionin 400 200 p < 0.0001 st 0.9515 V. -

E-Table 1. Drug Classification Category Name Generic Name
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Thorax e-Table 1. Drug classification Category Name Generic Name Antiplatelets clopidogrel, cilostazol, ticlopidine2, beraprost, beraprost–long acting, complavin Anticoagulants 2,3 dabigatran Statins1,2,3 atorvastatin2, simvastatin, pitavastatin, fluvastatin, pravastatin, rosuvastatin, amlodipine/atorvastatin Sodium channel blockers4,5† mexiletine, aprindine, cibenzoline Beta blocker acebutolol2 Class III antiarrhythmic drugs amiodarone1–6 Calcium channel blockers bepridil1, amlodipine/atorvastatin, telmisartan/amlodipine, valsartan/amlodipine, valsartan/cilnidipine, candesartan/amlodipine Angiotensin/converting enzyme enalapril inhibitor2‡ Thiazides trichlormethiazide, hydrochlorothiazide3,5, benzylhydrochlorothiazide/reserpine/carbazochrome, mefruside, telmisartan/hydrochlorothiazide, valsartan/hydrochlorothiazide, candesartan/hydrochlorothiazide, candesartan/trichlormethiazide, losartan/hydrochlorothiazide NSAIDs diclofenac2, celecoxib, loxoprofen, etodolac, nabumetone, pranoprofen Anti-rheumatics actarit, iguratimod, tofacitinib, penicillamine2–5, leflunomide1,3, sodium aurothiomalate2–6#, bucillamine Leukotriene receptor antagonist2* pranlukast 5-ASA mesalazine, salazosulfapyridine5 Tricyclic antidepressant Imipramine5, cromipramine, maprotiline Antiepileptics valproate, phenytoin2,3,5, ethotoin, carbamazepine2–5, zonisamide Interferon1,2,3 -

Genetic Factors Influencing Pyrimidine- Antagonist Chemotherapy
The Pharmacogenomics Journal (2005) 5, 226–243 & 2005 Nature Publishing Group All rights reserved 1470-269X/05 $30.00 www.nature.com/tpj REVIEW Genetic factors influencing Pyrimidine- antagonist chemotherapy JG Maring1 ABSTRACT 2 Pyrimidine antagonists, for example, 5-fluorouracil (5-FU), cytarabine (ara-C) HJM Groen and gemcitabine (dFdC), are widely used in chemotherapy regimes for 2 FM Wachters colorectal, breast, head and neck, non-small-cell lung cancer, pancreatic DRA Uges3 cancer and leukaemias. Extensive metabolism is a prerequisite for conversion EGE de Vries4 of these pyrimidine prodrugs into active compounds. Interindividual variation in the activity of metabolising enzymes can affect the extent of 1Department of Pharmacy, Diaconessen Hospital prodrug activation and, as a result, act on the efficacy of chemotherapy Meppel & Bethesda Hospital Hoogeveen, Meppel, treatment. Genetic factors at least partly explain interindividual variation in 2 The Netherlands; Department of Pulmonary antitumour efficacy and toxicity of pyrimidine antagonists. In this review, Diseases, University of Groningen & University Medical Center Groningen, Groningen, The proteins relevant for the efficacy and toxicity of pyrimidine antagonists will Netherlands; 3Department of Pharmacy, be summarised. In addition, the role of germline polymorphisms, tumour- University of Groningen & University Medical specific somatic mutations and protein expression levels in the metabolic Center Groningen, Groningen, The Netherlands; pathways and clinical pharmacology -

Appendix C Medication Tables
Appendix C Medication Tables Note: The medication tables are not meant to be inclusive lists of all available therapeutic agents. Approved medication tables will be updated regularly. Discrepancies must be reported. See Resource Section of this manual for additional contact information. Release Notes: Aspirin Table Version 1.0 Table 1.1 Aspirin and Aspirin-Containing Medications Acetylsalicylic Acid Acuprin 81 Alka-Seltzer Alka-Seltzer Morning Relief Anacin Arthritis Foundation Aspirin Arthritis Pain Ascriptin Arthritis Pain Formula ASA ASA Baby ASA Baby Chewable ASA Baby Coated ASA Bayer ASA Bayer Children's ASA Buffered ASA Children's ASA EC ASA Enteric Coated ASA/Maalox Ascriptin Aspergum Aspir-10 Aspir-Low Aspir-Lox Aspir-Mox Aspir-Trin Aspirbuf Aspircaf Aspirin Aspirin Baby Aspirin Bayer Aspirin Bayer Children's Aspirin Buffered Aspirin Child Aspirin Child Chewable Aspirin Children's Aspirin EC Aspirin Enteric Coated Specifications Manual for National Appendix C-1 Hospital Quality Measures Table 1.1 Aspirin and Aspirin-Containing Medications (continued) Aspirin Litecoat Aspirin Lo-Dose Aspirin Low Strength Aspirin Tri-Buffered Aspirin, Extended Release Aspirin/butalbital/caffeine Aspirin/caffeine Aspirin/pravachol Aspirin/pravastatin Aspirtab Bayer Aspirin Bayer Aspirin PM Extra Strength Bayer Children’s Bayer EC Bayer Enteric Coated Bayer Low Strength Bayer Plus Buffered ASA Buffered Aspirin Buffered Baby ASA Bufferin Bufferin Arthritis Strength Bufferin Extra Strength Buffex Cama Arthritis Reliever Child’s Aspirin Coated Aspirin -

Chemical Concentrations in Cell Culture Compartments (C5) – Free Concentrations
Kisitu et al.: Chemical Concentrations in Cell Culture Compartments (C5) – Free Concentrations Supplementary Data1 Table of contents Box S1: Concentrations then and now………………………………………………………………………………… 1 Derivation of the “extracellular biokinetics” formula…………………………………………………………………... 2 Box S2: Example use of Equation S13 with an acidic and basic drug……………………………………………… 7 Table S1: Cell composition data essential for biokinetics calculations…………………………………………….. 7 Table S2: Measured and predicted human plasma fu values………………………………………………………. 8 References………………………….…………………………………………………………………………………….. 13 Box S1: Concentrations then and now Dose: The concept of dose has been defined extensively before (Kisitu et al., 2019). It describes an absolute amount per experimental system (e.g., per mouse or per human patient). When the concept is applied to NAM, it describes the amount of chemical per cell culture well. Example, if a chemical concentration in the medium is 1 mM and the well contains 1 mL of medium, then the dose is 1 µmole; if the same well contains 2 mL medium, then the concentration is the same, but the dose doubles. Weight-normalized doses: Already in Paracelsus’ time it must have been clear that a dose tolerated by a tall and heavy adult may be lethal to a small child. This made clear that normalization to overall weight or volume is an important concept. Often normalized doses are expressed in dose per kg body weight (see Kisitu et al., 2019). Nominal concentration: If a dose in an in vitro system is normalized to the volume of the system, then a nominal concentration is obtained. This measure indicates what the drug/toxicant concentration would be if all chemical was freely dissolved and no losses/distribution occurred. -

(12) United States Patent (10) Patent No.: US 9,283,215 B2 Zeldis (45) Date of Patent: *Mar
US009283215 B2 (12) United States Patent (10) Patent No.: US 9,283,215 B2 Zeldis (45) Date of Patent: *Mar. 15, 2016 (54) METHODS FORTREATING MULTIPLE (2013.01); A61 K3I/4439 (2013.01); A61 K MYELOMAUSING 4-(AMINO)-2- 31/475 (2013.01); A61 K3I/515 (2013.01); (2,6-DIOXO(3-PIPERIDYL))-ISOINDOLINE- A6 IK3I/573 (2013.01); A61 K3I/675 SES COMBINATION WITH (2013.01); A61 K3I/704 (2013.01); A61 K 31/7048 (2013.01); A61 K35/12 (2013.01); (71) Applicant: Celgene Corporation, Summit, NJ (US) A61K39/00II (2013.01); A61 K39/3955 (2013.01); A61K 45/06 (2013.01); A61 K (72) Inventor: Jerome B. Zeldis, Princeton, NJ (US) 2300/00 (2013.01) (58) Field of Classification Search (73) Assignee: Celgene Corporation, Summit, NJ (US) CPC ........... A61K 31/4462: A61K 31/4035; A61 K (*) Notice: Subject to any disclaimer, the term of this 31f445 patent is extended or adjusted under 35 USPC .......................................... 514/323,329–330 U.S.C. 154(b) by 0 days. See application file for complete search history. This patent is Subject to a terminal dis claimer. (56) References Cited (21) Appl. No.: 14/201,069 U.S. PATENT DOCUMENTS (22) Filed: Mar. 7, 2014 3.536.809 A 10, 1970 Applezweig 3,598,123 A 8, 1971 Zaffaroni et al. O O 3,845,770 A 11/1974 Theeuwes et al. (65) Prior Publication Data 3,916,899 A 1 1/1975 Theeuwes et al. US 2014/0186404 A1 Jul. 3, 2014 4,008,719 A 2f1977 Theeuwes et al. 4,810,643 A 3, 1989 Souza Related U.S.