Phenotype Microarrays Panels PM-M1 to PM-M14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
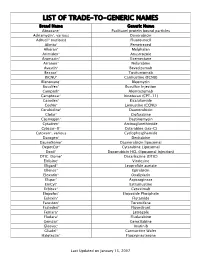
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Acla
[CANCER RESEARCH 49. 4357-4362, August 1. 1989] Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Aclarubicin Hydrochloride 1omonimi Ichihara,1 Kiyoshi Sakamoto, Katsutaka Mori, and Masanobu Akagi Department of Surgery II, Kumamoto University Medical School, Kumamoto 860, Japan 4BSTRACT MATERIALS AND METHODS Transcatheter arterial Chemoembolization therapy using polylactic Preparation of PLA-ACRms acid microspheres containing aclarubicin hydrochloride (ACR) was per PLA-ACRms were prepared in the pharmacy of the Kumamoto formed in 62 patients with primary hepatocellular carcinoma. These microspheres were about 200 ¡anin diameter and contained 10% (w/w) University Hospital. Briefly, aclarubicin hydrochloride and isopropyl aclarubicin. A single dose of polylactic acid microspheres containing ACR myristate, a medium-chain fatty acid ester, were dissolved in 7.5% (50-100 mg of ACR) was administered 1 to 8 times with a mean of 2.2 polylactic acid-methylene chloride. The resultant solution was dispersed in 1% gelatin solution and sterilized in an autoclave for 20 min at doses (a total of 160 treatments) in 62 patients. Antitumor effects were 120°C;thismixture was then stirred with a magnetic stirrer at 500 rpm observed from the decrease in serum a-fetoprotein levels (82.1% of the patients) and in two dimensional size of tumor on computed tomography for 1 h. The resultant microspheres were collected by filtration through (93.6%). The cumulative survival rate was 54.3% at 1 year, 24.6% at 2 a membrane filter (3 //m pore diameter), rinsed 2 to 3 times (in 1 liter years, and 19.2% at 3 years, respectively, among 59 patients with of distilled water), and dried under reduced pressure for 5 days. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
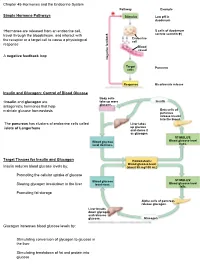
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

Identification of Repurposed Drugs for Chordoma Therapy
Identification of Repurposed Drugs for Chordoma Therapy. Menghang Xia, Ph.D. Division of Pre-Clinical Innovation National Center for Advancing Translational Sciences National Institutes of Health Fourth International Chordoma Research Workshop Boston, March 22, 2013 NIH Chemical Genomics Center Founded in 2004 • National Center for Advancing Translational Sciences (NCATS) • >100 staff: Biologists, Chemists, Informatics and Engineers Robotic HTS facility Mission • Development of chemical probes for novel biology • Novel targets, rare/neglected diseases • New technologies/paradigms for assay development, screening, informatics, chemistry Collaborations • >200 investigators worldwide • 60% NIH extramural • 25% NIH intramural • 15% Foundations, Research Consortia, Pharma/Biotech Steps in the drug development process Make Create Test modifications Test in Test in testing >100,000 to active animals for humans for system chemicals for chemicals to safety, safety, (aka, activity on make suitable effectiveness effectiveness “assay”) target for human use Two approaches to therapeutics for rare and neglected diseases 1-2 years? >400,000 compounds, 15 yrs Lead Preclinical Clinical Screen Hit Lead Optimization Development Trials 3500 drugs The NCGC Pharmaceutical Collection Procurement in Drug Source In house process Total US FDA* 1635 182 1817 UK/EU/Canada/Japan 756 177 933 Total Approved 2391 359 2750 Investigational 928 3953 4881 Total 3319 4312 7631 * These counts include approved veterinary drugs Informatics sources for NPC o US FDA: Orange Book, OTC, NDC, Green Book, Drugs at FDA o Britain NHS o EMEA o Health Canada o Japan NHI Physical sources for NPC o Procurement from >70 suppliers worldwide o In-house purification of APIs from marketed forms Drug plate composition o Synthesis Comprehensive Drug Repurposing Library Access to the NPC (http://tripod.nih.gov/npc/) Chordoma Screening Project • Cell lines Chordoma cell lines screened: U-CH1 and U-CH2B . -

Towards the Reduction of Occupational Exposure To
TOWARDS THE REDUCTION OF OCCUPATIONAL EXPOSURE TO CYTOTOXIC DRUGS by Cristian Vasile Barzan B.Sc. Simon Fraser University, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate Studies (Occupational and Environmental Hygiene) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2010 © Cristian Vasile Barzan, 2010 Abstract Background : One of the most powerful and widely used techniques in cancer treatment is the use of cytotoxic drugs in chemotherapy. These drugs are inherently hazardous with many of them causing carcinogenic, mutagenic or teratogenic health outcomes. Occupational exposure to cytotoxic drugs is of great concern due to their lack of selectivity between healthy and unhealthy cells. Widespread cytotoxic drug contamination has been reported in North America, Europe and Australia. Current cleaning protocols for hazardous antineoplastic drugs include the use of disinfectants and oxidizing agents, such as household bleach. Aim : The thesis project focused on two objectives: 1) hypothesize and confirm potential hazardous by- products arising from cleaning cyclophosphamide, a widely used cytotoxic drugs, with household bleach, a commonly used cleaning agent; 2) develop an effective and safe cleaning agent for cytotoxic drugs in order to prevent and eliminate exposure to these drugs. Methods : The gas chromatograph mass spectrum (GC/MS) was used to analyze the decomposition of cyclophosphamide by household bleach (5.25% hypochlorite). The reaction was conducted in a test-tube and the by-products extracted and derivatized prior to analysis. Multiple cleaning agent compositions were tested on 10x10cm stainless steel plates spiked with the two model cytotoxic drugs, cyclophosphamide and methotrexate. -

Comparative Genotoxicity of Adriamycin and Menogarol, Two Anthracycline Antitumor Agents
[CANCER RESEARCH 43, 5293-5297, November 1983] Comparative Genotoxicity of Adriamycin and Menogarol, Two Anthracycline Antitumor Agents B. K. Bhuyan,1 D. M. Zimmer, J. H. Mazurek, R. J. Trzos, P. R. Harbach, V. S. Shu, and M. A. Johnson Departments of Cancer Research [B. K. B.. D. M. Z.], Pathology and Toxicology Research [J. H. M., R. J. T., P. R. H.], and Biostatist/cs [V. S. S., M. A. J.], The Upjohn Company, Kalamazoo, Michigan 49001 ABSTRACT murine tumors such as P388 and L1210 leukemias and B16 melanoma (13). However, the biochemical activity of Adriamycin Adriamycin and menogarol are anthracyclines which cause and menogarol were markedly different in the following respects, more than 100% increase in life span of mice bearing P388 (a) at cytotoxic doses, Adriamycin inhibited RNA synthesis much leukemia and B16 melanoma. Unlike Adriamycin, menogarol more than DNA synthesis in L1210 cells in culture (10). In does not bind strongly to ONA, and it minimally inhibits DNA and contrast, menogarol caused very little inhibition of RNA or DNA RNA synthesis at lethal doses. Adriamycin is a clinically active synthesis at cytotoxic doses (10); (b) Adriamycin interacted drug, and menogarol is undergoing preclinical toxicology at Na strongly with DNA, in contrast to the weak interaction seen with tional Cancer Institute. In view of the reported mutagenicity of menogarol (10); (c) cells in S phase were most sensitive to Adriamycin, we have compared the genotoxicity of the two Adriamycin as compared to maximum toxicity of menogarol to drugs. Our results show that, although Adriamycin and meno cells in Gì(5).These results collectively suggested that meno garol differ significantly in their bacterial mutagenicity (Ames garol acts through some mechanism other than the intercalative assay), they have similar genotoxic activity in several mammalian DNA binding proposed for Adriamycin. -

Synthesis of Antitumor Fluorinated Pyrimidine Nucleosides
UOPP #1290994, VOL 49, ISS 2 Synthesis of Antitumor Fluorinated Pyrimidine Nucleosides Patrizia Ferraboschi, Samuele Ciceri, and Paride Grisenti QUERY SHEET This page lists questions we have about your paper. The numbers displayed at left can be found in the text of the paper for reference. In addition, please review your paper as a whole for correctness. There are no Editor Queries in this paper. TABLE OF CONTENTS LISTING The table of contents for the journal will list your paper exactly as it appears below: Synthesis of Antitumor Fluorinated Pyrimidine Nucleosides Patrizia Ferraboschi, Samuele Ciceri, and Paride Grisenti Organic Preparations and Procedures International, 49:1–85, 2017 Copyright Ó Taylor & Francis Group, LLC ISSN: 0030-4948 print / 1945-5453 online DOI: 10.1080/00304948.2017.1290994 Synthesis of Antitumor Fluorinated Pyrimidine Nucleosides Patrizia Ferraboschi, Samuele Ciceri, and Paride Grisenti Dipartimento di Biotecnologie Mediche e Medicina Traslazionale, Universita 5 degli Studi di Milano, Via Saldini 50, 20141 Milano, Italy Introduction Nucleosides, due to their biological role as constituents of nucleic acids, are main targets in the development of analogues aimed at antimetabolite-based therapy. Modified nucleo- sides can disrupt biological processes causing the death of cancer or virally-infected cells. 10 Fluorinated analogues of biologically active compounds are often characterized by a dramatic change in their activity, compared with the parent molecules. Fluorine, the most electronegative element, is isosteric with a hydroxy group, the C-F bond length (1.35 A) being similar to the C-O bond length (1.43 A). In addition, it is the second smallest atom and it can mimic hydrogen in a modified structure; its van der Waals radius (1.47 A) is intermedi- 15 ate between that of hydrogen (1.20 A) and that of oxygen (1.52 A). -
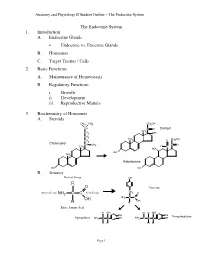
Lecture Outline
Anatomy and Physiology II Student Outline – The Endocrine System The Endocrine System 1. Introduction A. Endocrine Glands • Endocrine vs. Exocrine Glands B. Hormones C. Target Tissues / Cells 2. Basic Functions A. Maintenance of Homeostasis B. Regulatory Functions i. Growth ii. Development iii. Reproductive Matters 3. Biochemistry of Hormones A. Steroids CH3 CH3 CH2OH CH C O Cortisol CH CH2 3 OH CH2 CH CH OH CH 3 O 2 2 Cholesterol CH CH C O 3 H CH3 C HO HO CH 3 Aldosterone HO HO B. Amines Radical Group OH R O Tyrosine CH Amino Group NH2 C C Acid Group 2 O NH2 C C OH OH H H Basic Amino Acid H OH H H OH OH OH Norepinephrine Epinephrine NH2 C C OH NH2 C C C OH H H H H Page 1 Anatomy and Physiology II Student Outline – The Endocrine System C. Peptides • Antidiuretic Hormone • Oxytocin Oxytocin Antidiuretic Hormone Tyr Tyr Cys Cys Ileu Phe Glu Glu Cys Pro Leu Gly Cys Pro Arg Gly Asp Asp D. Proteins E. Glycoproteins 4. Feedback Control System A. Negative Feedback System (See Endocrine Pathways Handout: “Control Paradigm (Negative Feedback System)) i. Example: (See Endocrine Pathways Handout: “Negative Feedback Example”) B. Positive Feedback System (See Endocrine Pathways Handout: “Positive Feedback Example”) i. Child Birth and Oxytocin Page 2 Anatomy and Physiology II Student Outline – The Endocrine System 5. Mechanisms of Hormone Control A. Fixed-Membrane-Receptor Mechanism ii. Mechanism Inactive ATP Enzyme 1 Inactive cAMP Active Enzyme 2 Enzyme 1 (Secondary Active Messenger) Enzyme 2 Inactive Inactive Enzyme 4 ActiveEnzyme 3 Enzyme 3 Altered Active Cell Function Enzyme 4 ii. -

Functional Similarity of Anticancer Drugs by MTT Bioassay
cer Scien an ce C & f o T l h a e Hiwasa et al., J Cancer Sci Ther 2011, 3.10 n r a Journal of r p u 1000099 y o J DOI: 10.4172/1948-5956. ISSN: 1948-5956 Cancer Science & Therapy Rapid Communication Open Access Functional Similarity of Anticancer Drugs by MTT Bioassay Takaki Hiwasa1*,Takanobu Utsumi1, Mari Yasuraoka1, Nana Hanamura1, Hideaki Shimada2, Hiroshi Nakajima3, Motoo Kitagawa4, Yasuo Iwadate4,5, Ken-ichiro Goto6, Atsushi Takeda7, Kenzo Ohtsuka8, Hiroyoshi Ariga9 and Masaki Takiguchi1 1Department of Biochemistry, and Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 2Department of Surgery, School of Medicine, Toho University, Ota-ku, Tokyo 143-8541, Japan 3Department of Molecular Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 4Department of Molecular and Tumor Pathology, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 5Department of Neurological Surgery, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 6Department of Orthopaedic Surgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido, Chiba 284-0003, Japan 7Laboratory of Biochemistry, Graduate School of Nutritional Sciences, Sagami Women’s University, Sagamihara, Kanagawa 252-0383, Japan 8Laboratory of Cell & Stress Biology, Department of Environmental Biology, Chubu University, Matsumoto-cho, Kasugai, Aichi 487-8501, Japan 9Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo 060-0812, Japan Abstract We prepared normal or Ha-ras-transformed NIH3T3 cells transfected stably or transiently with various tumor- related genes. The chemosensitivity of the transfected clones to 16 anticancer drugs was compared to the parental control cells using the MTT assay. -

Cognitive Dysfunction
Cognitive Dysfunction Cancer, Chemotherapy and Cognitive Dysfunction Jörg Dietrich1 and Jochen Kaiser2 1. Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, US; 2. Institute of Medical Psychology, Medical Faculty, Goethe University, Frankfurt, Germany DOI: http://doi.org/10.17925/USN.2016.12.01.43 Abstract Decline in cognitive function, such as memory impairment, is one of the most commonly reported symptoms in cancer patients. Importantly, cognitive impairment is not restricted to patients treated for brain tumors, but also frequently present in patients treated for tumors outside the nervous system. Recent discoveries from preclinical and translational studies have defined various risk factors and mechanisms underlying such symptoms. The translation of these findings into clinical practice will improve patient management by limiting the degree of neurotoxicity from current therapies, and by exploring novel mechanisms of brain repair. Keywords Cognitive function, memory impairment, cancer, chemotherapy, radiation, neural progenitor cells Disclosure: Jörg Dietrich received support from the National Institute of Health, the American Academy of Neurology Foundation, and the American Cancer Society. Jörg Dietrich also received generous philanthropic support from the family foundations of Sheila McPhee, Ronald Tawil and Bryan Lockwood. Jochen Kaiser has nothing to declare in relation to this article. No funding was received for the publication of this article. Open Access: This article is published