Download This Topic [PDF]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Oncology and Breast Cancer
The Breast Center Smilow Cancer Hospital 20 York Street, North Pavilion New Haven, CT 06510 Phone: (203) 200-2328 Fax: (203) 200-2075 MEDICAL ONCOLOGY Treatment for breast cancer is multidisciplinary. The primary physicians with whom you may meet as part of your care are the medical oncologist, the breast surgeon, and often the radiation oncologist. A list of these specialty physicians will be provided to you. Each provider works with a team of caregivers to ensure that every patient receives high quality, personalized, breast cancer care. The medical oncologist specializes in “systemic therapy”, or medications that treat the whole body. For women with early stage breast cancer, systemic therapy is often recommended to provide the best opportunity to prevent breast cancer from returning. SYSTEMIC THERAPY Depending on the specific characteristics of your cancer, your medical oncologist may prescribe systemic therapy. Systemic therapy can be hormone pills, IV chemotherapy, antibody therapy (also called “immunotherapy”), and oral chemotherapy; sometimes patients receive more than one type of systemic therapy. Systemic therapy can happen before surgery (called “neoadjuvant therapy”) or after surgery (“adjuvant therapy”). If appropriate, your breast surgeon and medical oncologist will discuss the benefits of neoadjuvant and adjuvant therapy with you. As a National Comprehensive Cancer Network (NCCN) Member Institution, we are dedicated to following the treatment guidelines that have been shown to be most effective. We also have a variety of clinical trials that will help us find better ways to treat breast cancer. Your medical oncologist will recommend what treatment types and regimens are best for you. The information used to make these decisions include: the location of the cancer, the size of the cancer, the type of cancer, whether the cancer is invasive, the grade of the cancer (a measure of its aggressiveness), prognostic factors such as hormone receptors and HER2 status, and lymph node involvement. -
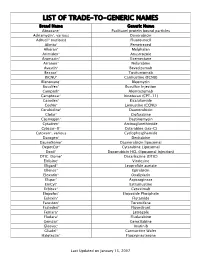
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

Exposure to Carcinogens and Work-Related Cancer: a Review of Assessment Methods
European Agency for Safety and Health at Work ISSN: 1831-9343 Exposure to carcinogens and work-related cancer: A review of assessment methods European Risk Observatory Report Exposure to carcinogens and work-related cancer: A review of assessment measures Authors: Dr Lothar Lißner, Kooperationsstelle Hamburg IFE GmbH Mr Klaus Kuhl (task leader), Kooperationsstelle Hamburg IFE GmbH Dr Timo Kauppinen, Finnish Institute of Occupational Health Ms Sanni Uuksulainen, Finnish Institute of Occupational Health Cross-checker: Professor Ulla B. Vogel from the National Working Environment Research Centre in Denmark Project management: Dr Elke Schneider - European Agency for Safety and Health at Work (EU-OSHA) Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers, or these calls may be billed. More information on the European Union is available on the Internet ( 48TU http://europa.euU48T). Cataloguing data can be found on the cover of this publication. Luxembourg: Publications Office of the European Union, 2014 ISBN: 978-92-9240-500-7 doi: 10.2802/33336 Cover pictures: (clockwise): Anthony Jay Villalon (Fotolia); ©Roman Milert (Fotolia); ©Simona Palijanskaite; ©Kari Rissa © European Agency for Safety and Health at Work, 2014 Reproduction is authorised provided the source is acknowledged. European Agency for Safety and Health at Work – EU-OSHA 1 Exposure to carcinogens and work-related cancer: -

About Ovarian Cancer Overview and Types
cancer.org | 1.800.227.2345 About Ovarian Cancer Overview and Types If you have been diagnosed with ovarian cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is Ovarian Cancer? Research and Statistics See the latest estimates for new cases of ovarian cancer and deaths in the US and what research is currently being done. ● Key Statistics for Ovarian Cancer ● What's New in Ovarian Cancer Research? What Is Ovarian Cancer? Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer and can spread. To learn more about how cancers start and spread, see What Is Cancer?1 Ovarian cancers were previously believed to begin only in the ovaries, but recent evidence suggests that many ovarian cancers may actually start in the cells in the far (distal) end of the fallopian tubes. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 What are the ovaries? Ovaries are reproductive glands found only in females (women). The ovaries produce eggs (ova) for reproduction. The eggs travel from the ovaries through the fallopian tubes into the uterus where the fertilized egg settles in and develops into a fetus. The ovaries are also the main source of the female hormones estrogen and progesterone. One ovary is on each side of the uterus. The ovaries are mainly made up of 3 kinds of cells. Each type of cell can develop into a different type of tumor: ● Epithelial tumors start from the cells that cover the outer surface of the ovary. -

Hydrazine Sulfate Hazard Summary Identification
Common Name: HYDRAZINE SULFATE CAS Number: 10034-93-2 RTK Substance number: 2360 DOT Number: None Date: December 1994 Revision: May 2001 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Hydrazine Sulfate can affect you when breathed in and * Exposure to hazardous substances should be routinely may be absorbed through the skin. evaluated. This may include collecting personal and area * Hydrazine Sulfate should be handled as a air samples. You can obtain copies of sampling results CARCINOGEN—WITH EXTREME CAUTION. from your employer. You have a legal right to this * Hydrazine Sulfate can irritate and burn the eyes and skin. information under OSHA 1910.1020. * Breathing Hydrazine Sulfate can irritate the nose, throat * If you think you are experiencing any work-related health and lungs causing coughing and shortness of breath. problems, see a doctor trained to recognize occupational * Exposure can cause you to feel dizzy and lightheaded. diseases. Take this Fact Sheet with you. Higher levels can cause trembling, a feeling of excitement, and even convulsions (fits). WORKPLACE EXPOSURE LIMITS * Hydrazine Sulfate may damage blood cells causing a low No occupational exposure limits have been established for blood count (anemia). Hydrazine Sulfate. This does not mean that this substance is * Exposure may damage the liver and kidneys. not harmful. Safe work practices should always be followed. * Hydrazine Sulfate may cause a skin allergy. If allergy develops, very low future exposure can cause itching and a * Hydrazine Sulfate may be a CARCINOGEN in humans. skin rash. There may be no safe level of exposure to a carcinogen, so all contact should be reduced to the lowest possible level. -

Primary Screening for Breast Cancer with Conventional Mammography: Clinical Summary
Primary Screening for Breast Cancer With Conventional Mammography: Clinical Summary Population Women aged 40 to 49 y Women aged 50 to 74 y Women aged ≥75 y The decision to start screening should be No recommendation. Recommendation Screen every 2 years. an individual one. Grade: I statement Grade: B Grade: C (insufficient evidence) These recommendations apply to asymptomatic women aged ≥40 y who do not have preexisting breast cancer or a previously diagnosed high-risk breast lesion and who are not at high risk for breast cancer because of a known underlying genetic mutation Risk Assessment (such as a BRCA1 or BRCA2 gene mutation or other familial breast cancer syndrome) or a history of chest radiation at a young age. Increasing age is the most important risk factor for most women. Conventional digital mammography has essentially replaced film mammography as the primary method for breast cancer screening Screening Tests in the United States. Conventional digital screening mammography has about the same diagnostic accuracy as film overall, although digital screening seems to have comparatively higher sensitivity but the same or lower specificity in women age <50 y. For women who are at average risk for breast cancer, most of the benefit of mammography results from biennial screening during Starting and ages 50 to 74 y. While screening mammography in women aged 40 to 49 y may reduce the risk for breast cancer death, the Stopping Ages number of deaths averted is smaller than that in older women and the number of false-positive results and unnecessary biopsies is larger. The balance of benefits and harms is likely to improve as women move from their early to late 40s. -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Hematopoietic and Lymphoid Neoplasm Coding Manual
Hematopoietic and Lymphoid Neoplasm Coding Manual Effective with Cases Diagnosed 1/1/2010 and Forward Published August 2021 Editors: Jennifer Ruhl, MSHCA, RHIT, CCS, CTR, NCI SEER Margaret (Peggy) Adamo, BS, AAS, RHIT, CTR, NCI SEER Lois Dickie, CTR, NCI SEER Serban Negoita, MD, PhD, CTR, NCI SEER Suggested citation: Ruhl J, Adamo M, Dickie L., Negoita, S. (August 2021). Hematopoietic and Lymphoid Neoplasm Coding Manual. National Cancer Institute, Bethesda, MD, 2021. Hematopoietic and Lymphoid Neoplasm Coding Manual 1 In Appreciation NCI SEER gratefully acknowledges the dedicated work of Drs, Charles Platz and Graca Dores since the inception of the Hematopoietic project. They continue to provide support. We deeply appreciate their willingness to serve as advisors for the rules within this manual. The quality of this Hematopoietic project is directly related to their commitment. NCI SEER would also like to acknowledge the following individuals who provided input on the manual and/or the database. Their contributions are greatly appreciated. • Carolyn Callaghan, CTR (SEER Seattle Registry) • Tiffany Janes, CTR (SEER Seattle Registry) We would also like to give a special thanks to the following individuals at Information Management Services, Inc. (IMS) who provide us with document support and web development. • Suzanne Adams, BS, CTR • Ginger Carter, BA • Sean Brennan, BS • Paul Stephenson, BS • Jacob Tomlinson, BS Hematopoietic and Lymphoid Neoplasm Coding Manual 2 Dedication The Hematopoietic and Lymphoid Neoplasm Coding Manual (Heme manual) and the companion Hematopoietic and Lymphoid Neoplasm Database (Heme DB) are dedicated to the hard-working cancer registrars across the world who meticulously identify, abstract, and code cancer data. -

Cancer-Related Terminology Used by a Healthcare Team
FEATURE IJPC GLossary OF THE MOST COMMON CANCER-RELated TERMINOLOGY USED BY A HEALthcare TEAM ADRENAL GLAND: Two glands, located near the kidneys, CANCER: A general term for more than 100 diseases in which ab- which produce hormones that control metabolism, fluid balance, normal cells grow out of control. Also used to refer to a malignant and blood pressure. They also produce small amounts of “male” (cancerous) tumor. hormones (androgens) and “female” hormones (estrogens and progesterone). CARCINOGEN: Any substance that causes cancer or helps cancer grow (e.g., tobacco smoke contains many carcinogens that greatly ADVANCED cancer: A general term describing later stages increase the risk of lung cancer) of cancer (usually stage 3 or 4) in which the disease has spread from the primary site (where it started) to other parts of the body. Catheter: A thin, flexible tube used to introduce fluids into the When the cancer has spread only to nearby areas, it is called locally body or to remove fluids from the body advanced. If it has spread to distant parts of the body, it is called metastatic. CELL: The basic unit of which all living things are made. Cells re- place themselves by dividing and forming new cells. The processes ALOPECIA: Hair loss, usually temporary. This is a side effect of that control the formation of new cells and the death of old cells are some chemotherapy drugs or radiation. disrupted in cancer. ANEMIA: Having too few red blood cells (“low blood”). This may CENTRAL VENOUS catheter (CVC): A special thin, flex- be a complication of the cancer or a side effect of treatment. -

Toxicological Profile for Hydrazines. US Department Of
TOXICOLOGICAL PROFILE FOR HYDRAZINES U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry September 1997 HYDRAZINES ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HYDRAZINES iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 HYDRAZINES vii CONTRIBUTORS CHEMICAL MANAGER(S)/AUTHOR(S): Gangadhar Choudhary, Ph.D. ATSDR, Division of Toxicology, Atlanta, GA Hugh IIansen, Ph.D. ATSDR, Division of Toxicology, Atlanta, GA Steve Donkin, Ph.D. Sciences International, Inc., Alexandria, VA Mr. Christopher Kirman Life Systems, Inc., Cleveland, OH THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying end points. 3. Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. HYDRAZINES ix PEER REVIEW A peer review panel was assembled for hydrazines. The panel consisted of the following members: 1. Dr. -

A Comparison Between Triplet and Doublet Chemotherapy in Improving
Guo et al. BMC Cancer (2019) 19:1125 https://doi.org/10.1186/s12885-019-6294-9 RESEARCH ARTICLE Open Access A comparison between triplet and doublet chemotherapy in improving the survival of patients with advanced gastric cancer: a systematic review and meta-analysis Xinjian Guo1, Fuxing Zhao1, Xinfu Ma1, Guoshuang Shen1, Dengfeng Ren1, Fangchao Zheng1,2,3, Feng Du4, Ziyi Wang1, Raees Ahmad1, Xinyue Yuan1, Junhui Zhao1* and Jiuda Zhao1* Abstract Background: Chemotherapy can improve the survival of patients with advanced gastric cancer. However, whether triplet chemotherapy can further improve the survival of patients with advanced gastric cancer compared with doublet chemotherapy remains controversial. This study reviewed and updated all published and eligible randomized controlled trials (RCTs) to compare the efficacy, prognosis, and toxicity of triplet chemotherapy with doublet chemotherapy in patients with advanced gastric cancer. Methods: RCTs on first-line chemotherapy in advanced gastric cancer on PubMed, Embase, and the Cochrane Register of Controlled Trials and all abstracts from the annual meetings of the European Society for Medical Oncology (ESMO) and the American Society of Clinical Oncology conferences up to October 2018 were searched. The primary outcome was overall survival, while the secondary outcomes were progression-free survival (PFS), time to progress (TTP), objective response rate (ORR), and toxicity. Results: Our analysis included 23 RCTs involving 4540 patients and 8 types of triplet and doublet chemotherapy regimens, and systematic review and meta-analysis revealed that triplet chemotherapy was superior compared with doublet chemotherapy in terms of improving median OS (HR = 0.92; 95% CI, 0.86–0.98; P = 0.02) and PFS (HR = 0.82; 95% CI, 0.69–0.97; P = 0.02) and TTP (HR = 0.92; 95% CI, 0.86–0.98; P = 0.02) and ORR (OR = 1.21; 95% CI, 1.12–1.31; P < 0.0001) among overall populations.