Plankton Microorganisms Coinciding with Two Consecutive Mass Fish Kills in a Newly Reconstructed Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Body Structure and Composition in the Apicomplexans Toxoplasma and Plasmodium Maria E
Francia et al. Cilia (2016) 5:3 DOI 10.1186/s13630-016-0025-5 Cilia REVIEW Open Access Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium Maria E. Francia1* , Jean‑Francois Dubremetz2 and Naomi S. Morrissette3 Abstract The phylum Apicomplexa encompasses numerous important human and animal disease-causing parasites, includ‑ ing the Plasmodium species, and Toxoplasma gondii, causative agents of malaria and toxoplasmosis, respectively. Apicomplexans proliferate by asexual replication and can also undergo sexual recombination. Most life cycle stages of the parasite lack flagella; these structures only appear on male gametes. Although male gametes (microgametes) assemble a typical 9 2 axoneme, the structure of the templating basal body is poorly defined. Moreover, the rela‑ tionship between asexual+ stage centrioles and microgamete basal bodies remains unclear. While asexual stages of Plasmodium lack defined centriole structures, the asexual stages of Toxoplasma and closely related coccidian api‑ complexans contain centrioles that consist of nine singlet microtubules and a central tubule. There are relatively few ultra-structural images of Toxoplasma microgametes, which only develop in cat intestinal epithelium. Only a subset of these include sections through the basal body: to date, none have unambiguously captured organization of the basal body structure. Moreover, it is unclear whether this basal body is derived from pre-existing asexual stage centrioles or is synthesized de novo. Basal bodies in Plasmodium microgametes are thought to be synthesized de novo, and their assembly remains ill-defined. Apicomplexan genomes harbor genes encoding δ- and ε-tubulin homologs, potentially enabling these parasites to assemble a typical triplet basal body structure. -

Periodic and Coordinated Gene Expression Between a Diazotroph and Its Diatom Host
The ISME Journal (2019) 13:118–131 https://doi.org/10.1038/s41396-018-0262-2 ARTICLE Periodic and coordinated gene expression between a diazotroph and its diatom host 1 1,2 1 3 4 Matthew J. Harke ● Kyle R. Frischkorn ● Sheean T. Haley ● Frank O. Aylward ● Jonathan P. Zehr ● Sonya T. Dyhrman1,2 Received: 11 April 2018 / Revised: 28 June 2018 / Accepted: 28 July 2018 / Published online: 16 August 2018 © International Society for Microbial Ecology 2018 Abstract In the surface ocean, light fuels photosynthetic carbon fixation of phytoplankton, playing a critical role in ecosystem processes including carbon export to the deep sea. In oligotrophic oceans, diatom–diazotroph associations (DDAs) play a keystone role in ecosystem function because diazotrophs can provide otherwise scarce biologically available nitrogen to the diatom host, fueling growth and subsequent carbon sequestration. Despite their importance, relatively little is known about the nature of these associations in situ. Here we used metatranscriptomic sequencing of surface samples from the North Pacific Subtropical Gyre (NPSG) to reconstruct patterns of gene expression for the diazotrophic symbiont Richelia and we – 1234567890();,: 1234567890();,: examined how these patterns were integrated with those of the diatom host over day night transitions. Richelia exhibited significant diel signals for genes related to photosynthesis, N2 fixation, and resource acquisition, among other processes. N2 fixation genes were significantly co-expressed with host nitrogen uptake and metabolism, as well as potential genes involved in carbon transport, which may underpin the exchange of nitrogen and carbon within this association. Patterns of expression suggested cell division was integrated between the host and symbiont across the diel cycle. -

Control of Phytoplankton Growth in Nutrient Recycling Ecosystems
MARINE ECOLOGY PROGRESS SERIES Published May 29 Mar. Ecol. Prog. Ser. Control of phytoplankton growth in nutrient recycling ecosystems. Theory and terminology T. Frede ~hingstadl,Egil sakshaug2 ' Department of Microbiology and Plant Physiology, University of Bergen, Jahnebk. 5,N-5007 Bergen, Norway Trondhjem Biological Station, The Museum. University of Trondheim, Bynesvn. 46, N-7018 Trondheim, Norway ABSTRACT: Some of the principles governing phytoplankton growth, biomass, and species composi- tion in 2-layered pelagic ecosystems are explored using an idealized, steady-state, mathematical model, based on simple extensions of Lotka-Volterra type equations. In particular, the properties of a food web based on 'small' and 'large' phytoplankton are investigated. Features of the phytoplankton community that may be derived from this conceptually simple model include CO-existenceof more than one species on one limiting nutrient, a rapid growth rate for a large fraction of the phytoplankton community in oligotrophic waters, a long food chain starting from a population of small phytoplankton in waters with moderate mixing over the nutricline, and a transition to dominance of a food chain based on large phytoplankton when mixing is increased. As pointed out by other authors, careless use of the concept of one limiting factor may be potentially confusing in such systems. To avoid this, a distinction in terminology between 'controlling' and 'limiting' factors is suggested. INTRODUCTION dominance of short food chains in upwelling areas (Ryther 1969). Various aspects of nutrient cycling in pelagic ecosys- Many authors have addressed various aspects of the tems are of prime importance to our understanding of planktonic ecosystem using mathematical models how productivity of the oceans is controlled, and to our which are idealized and conceptual. -
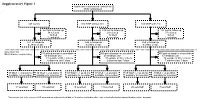
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Ellobiopsids of the Genus Thalassomyces Are Alveolates
J. Eukaryot. Microbiol., 51(2), 2004 pp. 246±252 q 2004 by the Society of Protozoologists Ellobiopsids of the Genus Thalassomyces are Alveolates JEFFREY D. SILBERMAN,a,b1 ALLEN G. COLLINS,c,2 LISA-ANN GERSHWIN,d,3 PATRICIA J. JOHNSONa and ANDREW J. ROGERe aDepartment of Microbiology, Immunology, and Molecular Genetics, University of California at Los Angeles, California, USA, and bInstitute of Geophysics and Planetary Physics, University of California at Los Angeles, California, USA, and cEcology, Behavior and Evolution Section, Division of Biology, University of California, La Jolla, California, USA, and dDepartment of Integrative Biology and Museum of Paleontology, University of California, Berkeley, California, USA, and eCanadian Institute for Advanced Research, Program in Evolutionary Biology, Genome Atlantic, Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia, Canada ABSTRACT. Ellobiopsids are multinucleate protist parasites of aquatic crustaceans that possess a nutrient absorbing `root' inside the host and reproductive structures that protrude through the carapace. Ellobiopsids have variously been af®liated with fungi, `colorless algae', and dino¯agellates, although no morphological character has been identi®ed that de®nitively allies them with any particular eukaryotic lineage. The arrangement of the trailing and circumferential ¯agella of the rarely observed bi-¯agellated `zoospore' is reminiscent of dino¯agellate ¯agellation, but a well-organized `dinokaryotic nucleus' has never been observed. Using small subunit ribosomal RNA gene sequences from two species of Thalassomyces, phylogenetic analyses robustly place these ellobiopsid species among the alveolates (ciliates, apicomplexans, dino¯agellates and relatives) though without a clear af®liation to any established alveolate lineage. Our trees demonstrate that Thalassomyces fall within a dino¯agellate 1 apicomplexa 1 Perkinsidae 1 ``marine alveolate group 1'' clade, clustering most closely with dino¯agellates. -

Diversity of Extracellular Proteins During the Transition from the 'Proto
1 Diversity of extracellular proteins during the transition from the ‘proto-apicomplexan’ alveolates to the apicomplexan obligate parasites THOMAS J. TEMPLETON1,2* and ARNAB PAIN3,4 1 Department of Protozoology, Institute of Tropical Medicine (NEKKEN), Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan 2 Department of Microbiology and Immunology, Weill Cornell Medical College, New York 10021, USA 3 Pathogen Genomics Laboratory, Biological and Environmental Sciences and Engineering (BESE) Division, King Abdullah University of Science and Technology (KAUST), Thuwal, Jeddah 23955-6900, Kingdom of Saudi Arabia 4 Global Station for Zoonosis Control, Global Institution for Collaborative Research and Education (GI-CoRE), Hokkaido University, N20 W10 Kita-ku, Sapporo 001-0020, Japan (Received 22 May 2015; revised 12 August 2015; accepted 14 August 2015) SUMMARY The recent completion of high-coverage draft genome sequences for several alveolate protozoans – namely, the chromer- ids, Chromera velia and Vitrella brassicaformis; the perkinsid Perkinsus marinus; the apicomplexan, Gregarina niphandrodes, as well as high coverage transcriptome sequence information for several colpodellids, allows for new genome-scale com- parisons across a rich landscape of apicomplexans and other alveolates. Genome annotations can now be used to help in- terpret fine ultrastructure and cell biology, and guide new studies to describe a variety of alveolate life strategies, such as symbiosis or free living, predation, and obligate intracellular parasitism, as well to provide foundations to dissect the evo- lutionary transitions between these niches. This review focuses on the attempt to identify extracellular proteins which might mediate the physical interface of cell–cell interactions within the above life strategies, aided by annotation of the repertoires of predicted surface and secreted proteins encoded within alveolate genomes. -

A Genomic Journey Through a Genus of Large DNA Viruses
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Virology Papers Virology, Nebraska Center for 2013 Towards defining the chloroviruses: a genomic journey through a genus of large DNA viruses Adrien Jeanniard Aix-Marseille Université David D. Dunigan University of Nebraska-Lincoln, [email protected] James Gurnon University of Nebraska-Lincoln, [email protected] Irina V. Agarkova University of Nebraska-Lincoln, [email protected] Ming Kang University of Nebraska-Lincoln, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/virologypub Part of the Biological Phenomena, Cell Phenomena, and Immunity Commons, Cell and Developmental Biology Commons, Genetics and Genomics Commons, Infectious Disease Commons, Medical Immunology Commons, Medical Pathology Commons, and the Virology Commons Jeanniard, Adrien; Dunigan, David D.; Gurnon, James; Agarkova, Irina V.; Kang, Ming; Vitek, Jason; Duncan, Garry; McClung, O William; Larsen, Megan; Claverie, Jean-Michel; Van Etten, James L.; and Blanc, Guillaume, "Towards defining the chloroviruses: a genomic journey through a genus of large DNA viruses" (2013). Virology Papers. 245. https://digitalcommons.unl.edu/virologypub/245 This Article is brought to you for free and open access by the Virology, Nebraska Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Virology Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Adrien Jeanniard, David D. Dunigan, James Gurnon, Irina V. Agarkova, Ming Kang, Jason Vitek, Garry Duncan, O William McClung, Megan Larsen, Jean-Michel Claverie, James L. Van Etten, and Guillaume Blanc This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ virologypub/245 Jeanniard, Dunigan, Gurnon, Agarkova, Kang, Vitek, Duncan, McClung, Larsen, Claverie, Van Etten & Blanc in BMC Genomics (2013) 14. -

Molecular Phylogeny and Surface Morphology of Colpodella Edax (Alveolata): Insights Into the Phagotrophic Ancestry of Apicomplexans
J. Eukaryot. Microbiol., 50(5), 2003 pp. 334±340 q 2003 by the Society of Protozoologists Molecular Phylogeny and Surface Morphology of Colpodella edax (Alveolata): Insights into the Phagotrophic Ancestry of Apicomplexans BRIAN S. LEANDER,a OLGA N. KUVARDINA,b VLADIMIR V. ALESHIN,b ALEXANDER P. MYLNIKOVc and PATRICK J. KEELINGa aCanadian Institute for Advanced Research, Program in Evolutionary Biology, Department of Botany, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada, and bDepartments of Evolutionary Biochemistry and Invertebrate Zoology, Belozersky Institute of Physico-Chemical Biology, Moscow State University, Moscow, 119 992, Russian Federation, and cInstitute for the Biology of Inland Waters, Russian Academy of Sciences, Borok, Yaroslavskaya oblast, 152742, Russian Federation ABSTRACT. The molecular phylogeny of colpodellids provides a framework for inferences about the earliest stages in apicomplexan evolution and the characteristics of the last common ancestor of apicomplexans and dino¯agellates. We extended this research by presenting phylogenetic analyses of small subunit rRNA gene sequences from Colpodella edax and three unidenti®ed eukaryotes published from molecular phylogenetic surveys of anoxic environments. Phylogenetic analyses consistently showed C. edax and the environmental sequences nested within a colpodellid clade, which formed the sister group to (eu)apicomplexans. We also presented surface details of C. edax using scanning electron microscopy in order to supplement previous ultrastructural investigations of this species using transmission electron microscopy and to provide morphological context for interpreting environmental sequences. The microscopical data con®rmed a sparse distribution of micropores, an amphiesma consisting of small polygonal alveoli, ¯agellar hairs on the anterior ¯agellum, and a rostrum molded by the underlying (open-sided) conoid. -

Genetic Diversity and Habitats of Two Enigmatic Marine Alveolate Lineages
AQUATIC MICROBIAL ECOLOGY Vol. 42: 277–291, 2006 Published March 29 Aquat Microb Ecol Genetic diversity and habitats of two enigmatic marine alveolate lineages Agnès Groisillier1, Ramon Massana2, Klaus Valentin3, Daniel Vaulot1, Laure Guillou1,* 1Station Biologique, UMR 7144, CNRS, and Université Pierre & Marie Curie, BP74, 29682 Roscoff Cedex, France 2Department de Biologia Marina i Oceanografia, Institut de Ciències del Mar, CMIMA, CSIC. Passeig Marítim de la Barceloneta 37-49, 08003 Barcelona, Spain 3Alfred Wegener Institute for Polar Research, Am Handelshafen 12, 27570 Bremerhaven, Germany ABSTRACT: Systematic sequencing of environmental SSU rDNA genes amplified from different marine ecosystems has uncovered novel eukaryotic lineages, in particular within the alveolate and stramenopile radiations. The ecological and geographic distribution of 2 novel alveolate lineages (called Group I and II in previous papers) is inferred from the analysis of 62 different environmental clone libraries from freshwater and marine habitats. These 2 lineages have been, up to now, retrieved exclusively from marine ecosystems, including oceanic and coastal waters, sediments, hydrothermal vents, and perma- nent anoxic deep waters and usually represent the most abundant eukaryotic lineages in environmen- tal genetic libraries. While Group I is only composed of environmental sequences (118 clones), Group II contains, besides environmental sequences (158 clones), sequences from described genera (8) (Hema- todinium and Amoebophrya) that belong to the Syndiniales, an atypical order of dinoflagellates exclu- sively composed of marine parasites. This suggests that Group II could correspond to Syndiniales, al- though this should be confirmed in the future by examining the morphology of cells from Group II. Group II appears to be abundant in coastal and oceanic ecosystems, whereas permanent anoxic waters and hy- drothermal ecosystems are usually dominated by Group I. -

Host-Microbe Relations: a Phylogenomics-Driven Bioinformatic Approach to the Characterization of Microbial DNA from Heterogeneous Sequence Data
Host-Microbe Relations: A Phylogenomics-Driven Bioinformatic Approach to the Characterization of Microbial DNA from Heterogeneous Sequence Data Timothy Patrick Driscoll Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Genetics, Bioinformatics, and Computational Biology Joseph J Gillespie David R Bevan Madhav V Marathe T M Murali May 1st, 2013 Blacksburg, Virginia Keywords: phylogenomics, genome-mining, host-microbe interactions, genomics, bioinformatics, symbiosis, bacteria, lateral gene transfer Copyright 2013 Host-Microbe Relations: A Phylogenomics-Driven Bioinformatic Approach to the Characterization of Microbial DNA from Heterogeneous Sequence Data Timothy Patrick Driscoll ABSTRACT Plants and animals are characterized by intimate, enduring, often indispensable, and always complex associations with microbes. Therefore, it should come as no surprise that when the genome of a eukaryote is sequenced, a medley of bacterial sequences are produced as well. These sequences can be highly informative about the interactions between the eukaryote and its bacterial cohorts; unfortunately, they often comprise a vanishingly small constituent within a heterogeneous mixture of microbial and host sequences. Genomic analyses typically avoid the bacterial sequences in order to obtain a genome sequence for the host. Metagenomic analysis typically avoid the host sequences in order to analyze community composition and functional diversity of the bacterial component. This dissertation describes the development of a novel approach at the intersection of genomics and metagenomics, aimed at the extraction and characterization of bacterial sequences from heterogeneous sequence data using phylogenomic and bioinformatic tools. To achieve this objective, three interoperable workflows were constructed as modular computational pipelines, with built-in checkpoints for periodic interpretation and refinement. -

Full-Length Transcriptome of Thalassiosira Weissflogii As
marine drugs Communication Full-Length Transcriptome of Thalassiosira weissflogii as a Reference Resource and Mining of Chitin-Related Genes Haomiao Cheng 1,2,3, Chris Bowler 4, Xiaohui Xing 5,6,7, Vincent Bulone 5,6,7, Zhanru Shao 1,2,* and Delin Duan 1,2,8,* 1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China; [email protected] 2 Laboratory for Marine Biology and Biotechnology, Pilot Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China 3 University of Chinese Academy of Sciences, Beijing 100049, China 4 Institut de Biologie de l’ENS (IBENS), Département de Biologie, École Normale Supérieure, CNRS, INSERM, Université PSL, 75005 Paris, France; [email protected] 5 Division of Glycoscience, Department of Chemistry, School of Engineering Sciences in Chemistry, Biotechnology and Health, Royal Institute of Technology (KTH), AlbaNova University Centre, 10691 Stockholm, Sweden; [email protected] (X.X.); [email protected] (V.B.) 6 Australian Research Council Centre of Excellence in Plant Cell Walls, School of Agriculture, Food and Wine, University of Adelaide, Waite Campus, Urrbrae 5064, Australia 7 Adelaide Glycomics, School of Agriculture Food and Wine, University of Adelaide, Waite Campus, Urrbrae 5064, Australia 8 State Key Laboratory of Bioactive Seaweed Substances, Qingdao Bright Moon Seaweed Group Co., Ltd., Qingdao 266400, China * Correspondence: [email protected] (Z.S.); [email protected] (D.D.) Citation: Cheng, H.; Bowler, C.; Xing, X.; Bulone, V.; Shao, Z.; Duan, D. Abstract: β-Chitin produced by diatoms is expected to have significant economic and ecological Full-Length Transcriptome of value due to its structure, which consists of parallel chains of chitin, its properties and the high Thalassiosira weissflogii as a Reference abundance of diatoms. -

Prasinophyceae)1
J. Phycol. 38, 1132–1142 (2002) DIEL VARIATIONS IN OPTICAL PROPERTIES OF MICROMONAS PUSILLA (PRASINOPHYCEAE)1 Michele D. DuRand,2 Rebecca E. Green, Heidi M. Sosik,3 and Robert J. Olson Woods Hole Oceanographic Institution, Biology Department, MS #32, Woods Hole, Massachusetts 02543, USA Micromonas pusilla (Butcher) Manton et Parke, a nitrogen ratio; Chli, intracellular chlorophyll concen- marine prasinophyte, was used to investigate how tration; CV, coefficient of variation; D, the diameter cell growth and division affect optical properties of of the mean cell; FLS, forward light scattering; G, phytoplankton over the light:dark cycle. Measurements geometric projected area of the mean cell; ␥, spectral were made of cell size and concentration, attenua- slope; , growth rate; n, real refractive index; nЈ, imag- tion and absorption coefficients, flow cytometric inary refractive index; a, absorption cross-section; forward and side light scattering and chl fluores- b, scattering cross-section; c, attenuation cross-sec- cence, and chl and carbon content. The refractive in- tion; Qa, efficiency factor for absorption; Qb, effi- dex was derived from observations and Mie scattering ciency factor for scattering; Qc, efficiency factor for theory. Diel variations occurred, with cells increasing attenuation; V , volume of the mean cell in size, light scattering, and carbon content during daytime photosynthesis and decreasing during night- time division. Cells averaged 1.6 m in diameter and exhibited phased division, with 1.3 divisions per day. Scattering changes resulted primarily from changes introduction in cell size and not refractive index; absorption Diel variations in bulk optical properties such as changes were consistent with a negligible package ef- beam attenuation (Siegel et al.