Diversity of Extracellular Proteins During the Transition from the 'Proto
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Basal Body Structure and Composition in the Apicomplexans Toxoplasma and Plasmodium Maria E
Francia et al. Cilia (2016) 5:3 DOI 10.1186/s13630-016-0025-5 Cilia REVIEW Open Access Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium Maria E. Francia1* , Jean‑Francois Dubremetz2 and Naomi S. Morrissette3 Abstract The phylum Apicomplexa encompasses numerous important human and animal disease-causing parasites, includ‑ ing the Plasmodium species, and Toxoplasma gondii, causative agents of malaria and toxoplasmosis, respectively. Apicomplexans proliferate by asexual replication and can also undergo sexual recombination. Most life cycle stages of the parasite lack flagella; these structures only appear on male gametes. Although male gametes (microgametes) assemble a typical 9 2 axoneme, the structure of the templating basal body is poorly defined. Moreover, the rela‑ tionship between asexual+ stage centrioles and microgamete basal bodies remains unclear. While asexual stages of Plasmodium lack defined centriole structures, the asexual stages of Toxoplasma and closely related coccidian api‑ complexans contain centrioles that consist of nine singlet microtubules and a central tubule. There are relatively few ultra-structural images of Toxoplasma microgametes, which only develop in cat intestinal epithelium. Only a subset of these include sections through the basal body: to date, none have unambiguously captured organization of the basal body structure. Moreover, it is unclear whether this basal body is derived from pre-existing asexual stage centrioles or is synthesized de novo. Basal bodies in Plasmodium microgametes are thought to be synthesized de novo, and their assembly remains ill-defined. Apicomplexan genomes harbor genes encoding δ- and ε-tubulin homologs, potentially enabling these parasites to assemble a typical triplet basal body structure. -

Exploration of Laboratory Techniques Relating to Cryptosporidium Parvum Propagation, Life Cycle Observation, and Host Immune Responses to Infection
EXPLORATION OF LABORATORY TECHNIQUES RELATING TO CRYPTOSPORIDIUM PARVUM PROPAGATION, LIFE CYCLE OBSERVATION, AND HOST IMMUNE RESPONSES TO INFECTION A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Cheryl Marie Brown In Partial Fulfillment for the Degree of MASTER OF SCIENCE Major Department: Veterinary and Microbiological Sciences February 2014 Fargo, North Dakota North Dakota State University Graduate School Title EXPLORATION OF LABORATORY TECHNIQUES RELATING TO CRYPTOSPORIDIUM PARVUM PROPAGATION, LIFE CYCLE OBSERVATION, AND HOST IMMUNE RESPONSES TO INFECTION By Cheryl Marie Brown The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of MASTER OF SCIENCE SUPERVISORY COMMITTEE: Dr. Jane Schuh Chair Dr. John McEvoy Dr. Carrie Hammer Approved: 4-8-14 Dr. Charlene Wolf-Hall Date Department Chair ii ABSTRACT Cryptosporidium causes cryptosporidiosis, a self-limiting diarrheal disease in healthy people, but causes serious health issues for immunocompromised individuals. Cryptosporidiosis has been observed in humans since the early 1970s and continues to cause public health concerns. Cryptosporidium has a complicated life cycle making laboratory study challenging. This project explores several ways of studying Cryptosporidium parvum, with a goal of applying existing techniques to further understand this life cycle. Utilization of a neonatal mouse model demonstrated laser microdissection as a tool for studying host immune response to infeciton. A cell culture technique developed on FrameSlides™ enables laser microdissection of individual infected cells for further analysis. Finally, the hypothesis that the availability of cells to infect drives the switch from asexual to sexual parasite reproduction was tested by time-series infection. -

Cryptosporidium and Water
Cryptosporidium and Water: A Public Health Handbook 1997 WG WCWorking Group on Waterborne Cryptosporidiosis Suggested Citation Cryptosporidium and Water: A Public Health Handbook. Atlanta, Georgia: Working Group on Waterborne Cryptosporidiosis. CDCENTERS FOR DISEASEC CONTROL AND PREVENTION For additional copies of this handbook, write to: Centers for Disease Control and Prevention National Center for Infectious Diseases Division of Parasitic Diseases Mailstop F-22 4770 Buford Highway N.E. Atlanta, GA 30341-3724 CONTENTS Executive Summary Introduction 1- Coordination and Preparation 2- Epidemiologic Surveillance 3- Clinical Laboratory Testing 4- Evaluating Water Test Results Drinking Water Sources, Treatment, and Testing Environmental Sampling Methods Issuing and Rescinding a Boil Water Advisory 5- Outbreak Management Outbreak Assessment News Release Information Frequently Asked Questions Protocols for Special Audiences and Contingencies 6- Educational Information Preventing Cryptosporidiosis: A Guide for Persons With HIV and AIDS Preventing Cryptosporidiosis: A Guide for the Public Preventing Cryptosporidiosis: A Guide to Water Filters and Bottled Water 7- Recreational Water Appendix Selected Articles Key Words and Phrases Figures A-F Index Working Group on Waterborne Cryptosporidiosis (WGWC) Daniel G. Colley and Dennis D. Juranek, Coordinators, WGWC Division of Parasitic Diseases (DPD) National Center for Infectious Diseases Centers for Disease Control and Prevention Scott A. Damon, Publications Coordinator, WGWC, Centers for Disease Control and Prevention Margaret Hurd, Communications Coordinator, WGWC, Centers for Disease Control and Prevention Mary E. Bartlett, DPD Editor, Centers for Disease Control and Prevention Leslie S. Parker, Visual Information Specialist, Centers for Disease Control and Prevention Task Forces and Other Contributors: The draft materials for this handbook were developed through the work of multiple task forces and individuals whose names appear at the beginning of each chapter/section. -

University of Oklahoma
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By JOSHUA THOMAS COOPER Norman, Oklahoma 2017 MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________ Dr. Boris Wawrik, Chair ______________________________ Dr. J. Phil Gibson ______________________________ Dr. Anne K. Dunn ______________________________ Dr. John Paul Masly ______________________________ Dr. K. David Hambright ii © Copyright by JOSHUA THOMAS COOPER 2017 All Rights Reserved. iii Acknowledgments I would like to thank my two advisors Dr. Boris Wawrik and Dr. J. Phil Gibson for helping me become a better scientist and better educator. I would also like to thank my committee members Dr. Anne K. Dunn, Dr. K. David Hambright, and Dr. J.P. Masly for providing valuable inputs that lead me to carefully consider my research questions. I would also like to thank Dr. J.P. Masly for the opportunity to coauthor a book chapter on the speciation of diatoms. It is still such a privilege that you believed in me and my crazy diatom ideas to form a concise chapter in addition to learn your style of writing has been a benefit to my professional development. I’m also thankful for my first undergraduate research mentor, Dr. Miriam Steinitz-Kannan, now retired from Northern Kentucky University, who was the first to show the amazing wonders of pond scum. Who knew that studying diatoms and algae as an undergraduate would lead me all the way to a Ph.D. -

(Alveolata) As Inferred from Hsp90 and Actin Phylogenies1
J. Phycol. 40, 341–350 (2004) r 2004 Phycological Society of America DOI: 10.1111/j.1529-8817.2004.03129.x EARLY EVOLUTIONARY HISTORY OF DINOFLAGELLATES AND APICOMPLEXANS (ALVEOLATA) AS INFERRED FROM HSP90 AND ACTIN PHYLOGENIES1 Brian S. Leander2 and Patrick J. Keeling Canadian Institute for Advanced Research, Program in Evolutionary Biology, Departments of Botany and Zoology, University of British Columbia, Vancouver, British Columbia, Canada Three extremely diverse groups of unicellular The Alveolata is one of the most biologically diverse eukaryotes comprise the Alveolata: ciliates, dino- supergroups of eukaryotic microorganisms, consisting flagellates, and apicomplexans. The vast phenotypic of ciliates, dinoflagellates, apicomplexans, and several distances between the three groups along with the minor lineages. Although molecular phylogenies un- enigmatic distribution of plastids and the economic equivocally support the monophyly of alveolates, and medical importance of several representative members of the group share only a few derived species (e.g. Plasmodium, Toxoplasma, Perkinsus, and morphological features, such as distinctive patterns of Pfiesteria) have stimulated a great deal of specula- cortical vesicles (syn. alveoli or amphiesmal vesicles) tion on the early evolutionary history of alveolates. subtending the plasma membrane and presumptive A robust phylogenetic framework for alveolate pinocytotic structures, called ‘‘micropores’’ (Cavalier- diversity will provide the context necessary for Smith 1993, Siddall et al. 1997, Patterson -

Cryptosporidiosis (Last Updated July 16, 2019; Last Reviewed July 16, 2019)
Cryptosporidiosis (Last updated July 16, 2019; last reviewed July 16, 2019) Epidemiology Cryptosporidiosis is caused by various species of the protozoan parasite Cryptosporidium, which infect the small bowel mucosa, and, if symptomatic, the infection typically causes diarrhea. Cryptosporidium can also infect other gastrointestinal and extraintestinal sites, especially in individuals whose immune systems are suppressed. Advanced immunosuppression—typically CD4 T lymphocyte cell (CD4) counts <100 cells/ mm3—is associated with the greatest risk for prolonged, severe, or extraintestinal cryptosporidiosis.1 The three species that most commonly infect humans are Cryptosporidium hominis, Cryptosporidium parvum, and Cryptosporidium meleagridis. Infections are usually caused by one species, but a mixed infection is possible.2,3 Cryptosporidiosis remains a common cause of chronic diarrhea in patients with AIDS in developing countries, with up to 74% of diarrheal stools from patients with AIDS demonstrating the organism.4 In developed countries with low rates of environmental contamination and widespread availability of potent antiretroviral therapy, the incidence of cryptosporidiosis has decreased. In the United States, the incidence of cryptosporidiosis in patients with HIV is now <1 case per 1,000 person-years.5 Infection occurs through ingestion of Cryptosporidium oocysts. Viable oocysts in feces can be transmitted directly through contact with humans or animals infected with Cryptosporidium, particularly those with diarrhea. Cryptosporidium oocysts can contaminate recreational water sources, such as swimming pools and lakes, and public water supplies and may persist despite standard chlorination. Person-to-person transmission of Cryptosporidium is common, especially among sexually active men who have sex with men. Clinical Manifestations Patients with cryptosporidiosis most commonly have acute or subacute onset of watery diarrhea, which may be accompanied by nausea, vomiting, and lower abdominal cramping. -

Control of Intestinal Protozoa in Dogs and Cats
Control of Intestinal Protozoa 6 in Dogs and Cats ESCCAP Guideline 06 Second Edition – February 2018 1 ESCCAP Malvern Hills Science Park, Geraldine Road, Malvern, Worcestershire, WR14 3SZ, United Kingdom First Edition Published by ESCCAP in August 2011 Second Edition Published in February 2018 © ESCCAP 2018 All rights reserved This publication is made available subject to the condition that any redistribution or reproduction of part or all of the contents in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise is with the prior written permission of ESCCAP. This publication may only be distributed in the covers in which it is first published unless with the prior written permission of ESCCAP. A catalogue record for this publication is available from the British Library. ISBN: 978-1-907259-53-1 2 TABLE OF CONTENTS INTRODUCTION 4 1: CONSIDERATION OF PET HEALTH AND LIFESTYLE FACTORS 5 2: LIFELONG CONTROL OF MAJOR INTESTINAL PROTOZOA 6 2.1 Giardia duodenalis 6 2.2 Feline Tritrichomonas foetus (syn. T. blagburni) 8 2.3 Cystoisospora (syn. Isospora) spp. 9 2.4 Cryptosporidium spp. 11 2.5 Toxoplasma gondii 12 2.6 Neospora caninum 14 2.7 Hammondia spp. 16 2.8 Sarcocystis spp. 17 3: ENVIRONMENTAL CONTROL OF PARASITE TRANSMISSION 18 4: OWNER CONSIDERATIONS IN PREVENTING ZOONOTIC DISEASES 19 5: STAFF, PET OWNER AND COMMUNITY EDUCATION 19 APPENDIX 1 – BACKGROUND 20 APPENDIX 2 – GLOSSARY 21 FIGURES Figure 1: Toxoplasma gondii life cycle 12 Figure 2: Neospora caninum life cycle 14 TABLES Table 1: Characteristics of apicomplexan oocysts found in the faeces of dogs and cats 10 Control of Intestinal Protozoa 6 in Dogs and Cats ESCCAP Guideline 06 Second Edition – February 2018 3 INTRODUCTION A wide range of intestinal protozoa commonly infect dogs and cats throughout Europe; with a few exceptions there seem to be no limitations in geographical distribution. -

Genetic Basis for Virulence Differences of Various Cryptosporidium Parvum Carcinogenic Isolates
Genetic basis for virulence differences of various Cryptosporidium parvum carcinogenic isolates Christophe Audebert, Franck Bonardi, Ségolène Caboche, Karine Guyot, Hélène Touzet, Sophie Merlin, Nausicaa Gantois, Colette Creusy, Dionigia Meloni, Anthony Mouray, et al. To cite this version: Christophe Audebert, Franck Bonardi, Ségolène Caboche, Karine Guyot, Hélène Touzet, et al.. Ge- netic basis for virulence differences of various Cryptosporidium parvum carcinogenic isolates. Scientific Reports, Nature Publishing Group, 2020, 10 (1), pp.7316. 10.1038/s41598-020-64370-0. inserm- 02873228 HAL Id: inserm-02873228 https://www.hal.inserm.fr/inserm-02873228 Submitted on 18 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. www.nature.com/scientificreports OPEN Genetic basis for virulence diferences of various Cryptosporidium parvum carcinogenic isolates Christophe Audebert1,2, Franck Bonardi3, Ségolène Caboche 2,4, Karine Guyot4, Hélène Touzet3,5, Sophie Merlin1,2, Nausicaa Gantois4, Colette Creusy6, Dionigia Meloni4, Anthony Mouray7, Eric Viscogliosi4, Gabriela Certad4,8, Sadia Benamrouz-Vanneste4,9 & Magali Chabé 4 ✉ Cryptosporidium parvum is known to cause life-threatening diarrhea in immunocompromised hosts and was also reported to be capable of inducing digestive adenocarcinoma in a rodent model. Interestingly, three carcinogenic isolates of C. -

Epidemiology of Cryptosporidiosis in HIV-Infected Individuals
Iqbal et al., 1:9 http://dx.doi.org/10.4172/scientificreports.431 Open Access Open Access Scientific Reports Scientific Reports Review Article OpenOpen Access Access Epidemiology of Cryptosporidiosis in HIV-Infected Individuals: A Global Perspective Asma Iqbal1,2, Yvonne AL Lim1*, Mohammad AK Mahdy1, Brent R Dixon2 and Johari Surin2 1Department of Parasitology, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia 2Bureau of Microbial Hazards, Food Directorate, Health Canada, Banting Research Centre, 251 Sir Frederick Banting Driveway, P.L.2204E, Ottawa, Ontario, K1A 0K9 Canada Abstract Cryptosporidiosis, resulting from infection with the protozoan parasite Cryptosporidium, is a significant opportunistic disease among HIV-infected individuals. With multiple routes of infection due to the recalcitrant nature of its infectious stage in the environment, the formulation of effective and practical control strategies for cryptosporidiosis must be based on a firm understanding of its epidemiology. Prevalence data and molecular characterization of Cryptosporidium in HIV-infected individuals is currently available from numerous countries in Africa, Asia, Europe, North America and South America, and it is clear that significant differences exist between developing and developed regions. This review highlights the current global status of Cryptosporidium infections among HIV-infected individuals, and puts forth a contextual framework for the development of integrated surveillance and control programs for cryptosporidiosis in immune compromised patients. Given that there are few specific chemotherapeutic agents available for cryptosporidiosis, and therapy is largely based on improving the patient’s immune status, the focus should be on patient compliance and non-compliance obstacles to the effective delivery of health care, as well as educating HIV-infected individuals in the prevention of infection. -

Cryptosporidium Hominis N. Sp. (Apicomplexa: Cryptosporidiidae) from Homo Sapiens
The Journal of Eukaryotic Microbiology Volume 49 November-December 2002 Number 6 J. Eukiiyor rMioohio/.. 49(6). 2002 pp. 433440 0 2002 by the Society oi Protozoologisls Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens UNA M. MORGAN-RYAN,” ABBIE FALL; LUCY A. WARD? NAWAL HIJJAWI: IRSHAD SULAIMAN: RONALD FAYER,” R. C. ANDREW THOMPSON,” M. OLSON,’ ALTAF LAL‘ and LIHUA XUOC “Division of Veterinory and Biomedical Sciences, Murdoch University, Murdoch, Western Australia 6150, and bFood Animal Health Research Program and Department of Veterinuq Preveittative Medicine, Ohio Agricultural Research and Development Centre, The Ohio State University, Wooster, Ohio 44691 USA, rind ‘Division of Parasitic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Public Health Services, U. S. Department of Health and Huniun Services, Atlantu, Georgia 30341. and W. S. Department of Agriculture, Anitnal Waste Pathogen Laboratory, Beltsville, Maryland 20705, und eUniver.~ir)of Calgary., FaculQ of Medicine, Animal Resources Center, Department OJ‘ Gastrointestinal Science, 3330 Hospital DI-NW, Calgary, Albertu 72N 4NI, Canada ABSTRACT. The structure and infectivity of the oocysts of a new species of Cryptosporidiurn from the feces of humans are described. Oocysts are structurally indistinguishable from those of Cr.y~~tos€,oridiunlpurvum. Oocysts of the new species are passed fully sporulated, lack sporocysts, and measure 4.4-5.4 prn (mean = 4.86) X 4.4-5.9 pm (mean = 5.2 prn) with a length to width ratio 1 .&I .09 (mean 1.07) (n = 100). Oocysts were not infectious for ARC Swiss mice. nude mice, Wistat’ rat pups, puppies, kittens or calves, but were infectious to neonatal gnotobiotic pigs. -

High Frequency of Cryptosporidium Hominis Infecting Infants Points to a Potential Anthroponotic Transmission in Maputo, Mozambique
pathogens Brief Report High Frequency of Cryptosporidium hominis Infecting Infants Points to A Potential Anthroponotic Transmission in Maputo, Mozambique Idalécia Cossa-Moiane 1,2,* , Hermínio Cossa 3, Adilson Fernando Loforte Bauhofer 1,4 , Jorfélia Chilaúle 1, Esperança Lourenço Guimarães 1,4, Diocreciano Matias Bero 1 , Marta Cassocera 1,4, Miguel Bambo 1, Elda Anapakala 1, Assucênio Chissaque 1,4 ,Júlia Sambo 1,4, Jerónimo Souzinho Langa 1, Lena Vânia Manhique-Coutinho 1, Maria Fantinatti 5, Luis António Lopes-Oliveira 5, Alda Maria Da-Cruz 5,6 and Nilsa de Deus 1,7 1 Instituto Nacional de Saúde (INS), EN1, Bairro da Vila–Parcela n◦ 3943, Distrito de Marracuene, Maputo 264, Mozambique; [email protected] (A.F.L.B.); [email protected] (J.C.); [email protected] (E.L.G.); [email protected] (D.M.B.); [email protected] (M.C.); [email protected] (M.B.); [email protected] (E.A.); [email protected] (A.C.); [email protected] (J.S.); [email protected] (J.S.L.); [email protected] (L.V.M.-C.); [email protected] (N.d.D.) 2 Institute of Tropical Medicine, 2000 Antwerp, Belgium 3 Centro de Investigação em Saúde de Manhiça (CISM), Unidade de Pesquisa Social, Manhiça Foundation (Fundação Manhiça, FM), Manhiça 1929, Mozambique; [email protected] Citation: Cossa-Moiane, I.; Cossa, H.; 4 Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, 1349-008 Lisboa, Portugal 5 Bauhofer, A.F.L.; Chilaúle, J.; Laboratório Interdisciplinar de Pesquisas Médicas, Instituto Oswaldo Cruz-FIOCRUZ, Guimarães, E.L.; Bero, D.M.; Rio de Janeiro 22040-360, Brazil; [email protected] (M.F.); [email protected] (L.A.L.-O.); alda@ioc.fiocruz.br (A.M.D.-C.) Cassocera, M.; Bambo, M.; Anapakala, 6 Disciplina de Parasitologia, Faculdade de Ciências Médicas, UERJ/RH, Rio de Janeiro 21040-900, Brazil E.; Chissaque, A.; et al. -
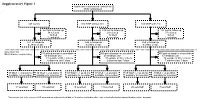
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines.