September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. Joe Shia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOOTROPIL® Piracetam
NOOTROPIL® Piracetam QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 800 mg or 1200 mg of piracetam. Each ml of oral solution contains 200 mg of piracetam EXCIPIENTS NOOTROPIL 800 mg and 1200 mg film-coated tablet: Core: Macrogol 6000 - Colloidal anhydrous silica - Magnesium stearate - Sodium croscarmellose Film-coating: Hydroxypropylmethylcellulose - Titanium dioxide (E171) - Macrogol 400 - Macrogol 6000. NOOTROPIL 200 mg/ml oral solution: Glycerol (85%) - Saccharin sodium - Apricot flavour - Caramel flavour - Methyl parahydroxybenzoate - Propyl parahydroxybenzoate - Sodium acetate - Glacial acetic acid - Purified water. PHARMACEUTICAL FORM NOOTROPIL Tablet 800 and 1200 mg: white, oblong, film-coated tablet, with a bisect line, marked N/N on one side and plain on the other side NOOTROPIL Oral Solution 20%: clear colourless solution INDICATIONS 1. Studies carried out in the elderly suffering from loss of memory, vertigo, a lack of concentration or of alertness, changes of mood, a deterioration in behaviour and personal negligence, demonstrate an improvement in symptoms. These symptoms can also provide an early warning of the onset of pathological ageing such as Alzheimer’s Disease, an Alzheimer type of senile dementia, or the dementia produced by multiple cerebral infarcts. 2. NOOTROPIL is advocated in the treatment of sickle-cell vaso-occlusive crises. 3. Studies have shown some improvement in children with learning difficulties associated with the written word, particularly with textual understanding which cannot be explained by intellectual backwardness, inadequate education or by the family environment. The administration of NOOTROPIL does not replace other measures also well adapted to correct these learning difficulties, such as remedial teaching. DOSAGE AND ADMINISTRATION Oral formulations 1 NOOTROPIL should be administered orally, and may be taken with or without food. -

Aniracetam Reduces Glutamate Receptor Desensitization and Slows
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 10936-10940, December 1991 Neurobiology Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus (non-N-methyl-D-aspartate receptor/synapse) JEFFRY S. ISAACSON*t AND ROGER A. NICOLLtt *Physiology Graduate Program and the Departments of SPharmacology and tPhysiology, University of California, San Francisco, CA 94143-0450 Communicated by Floyd E. Bloom, September 16, 1991 ABSTRACT Aniracetam is a nootropic drug that has been and DL-2-amino-5-phosphonovaleric acid (50 ,uM) were shown to selectively enhance quisqualate receptor-mediated added to the medium to block y-aminobutyric acid type A responses inXenopus oocytes injected with brain mRNA and in (GABAA) receptors and NMDA receptors, respectively. In hippocampal pyramidal cells [Ito, I., Tanabe, S., Kohda, A. & the majority of experiments examining iontophoretic re- Sugiyama, H. (1990) J. Physiol. (London) 424, 533-544]. We sponses, tetrodotoxin (0.5-1 1uM) was included to block have used patch clamp recording techniques in hippocampal sodium-dependent action potentials. Currents were recorded slices to elucidate the mechanism for this selective action. We with an Axopatch 1B amplifier from neurons in the CA1 and find that aniracetam enhances glutamate-evoked currents in CA3 pyramidal cell layers and granule cell layer of the whole-cell recordings and, in outside-out patches, strongly dentate gyrus using the "blind" whole-cell recording tech- reduces glutamate receptor desensitization. In addition, nique (15, 16). Patch electrodes (tip diameter = 2 Ium) aniracetam selectively prolongs the time course and increases contained (in mM) either a CsF (110 CsF, 10 CsCl, 10 Hepes, the peak amplitude of fast synaptic currents. -

Oral Fluid Drug Test Package Insert
Marijuana (THC) 11-nor-Δ9-THC-9 COOH 4 MTD: Methadone is a synthetic analgesic drug originally used for the Oral Fluid Drug Test treatment of narcotic addiction. In addition to use as a narcotic agonist, methadone is being used more frequently as a pain management agent. The Marijuana (THC) Δ9-THC 50 Package Insert psychological effects induced by using methadone are analgesia, sedation, and respiratory depression. Based on the saliva/plasma ratio calculated over Package insert for testing of the following drugs: > 0.02 % Alcohol (ALC) Alcohol salivary pH ranges of 6.4-7.6 for therapeutic or recreational doses of Amphetamine,Cocaine,Marijuana,Methamphetamine,Opiate,Methadone, B.A.C methadone, a cut-off <50 ng/mL is suggested. Due to this recommendation, Phencyclidine,Oxycodone,Benzodiazepine,Buprenorphine,Barbiturates, the cut-off level of the methadone test was calibrated to 30 ng/mL. Cotinine,EDDP,MDMA,6-MAM,Propoxyphene ,Fentanyl,ETG and Alcohol Tramadol(TRA) 50 Tramadol PCP: Phencyclidine is a hallucinogen and, can be detected in oral fluid as a INTENDED USE & SUMMARY result of the exchange of the drug between the circulatory system and the oral cavity.5 Fentanyl(FEN) 10 The Oral Fluid Drug And Alcohol Test is intended for screening for the Norfentanyl presence of drugs and alcohol and their metabolites in oral fluid. For professional in vitro diagnostic use only. OXY: Oxycodone is a semi-synthetic opioid with a structural similarity to Ethyl codeine. The drug is manufactured by modifying thebaine, an alkaloid found in 150 The Oral Fluid Pipette Test is a lateral flow chromatographic immunoassay for Glucuronide(ETG) the opium poppy. -

Review Memorandum
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k062165 B. Purpose for Submission: New device C. Measurand: Barbiturates D. Type of Test: Qualitative and semi-quantitative enzyme immunoassay E. Applicant: Ortho-Clinical Diagnostics, Inc. F. Proprietary and Established Names: VITROS Chemistry Products BARB Reagent VITROS Chemistry Products Calibrator 26 VITROS Chemistry Products FS Calibrator 1 VITROS Chemistry Products DAT Performance Verifiers I, II, III, IV and V G. Regulatory Information: 1. Regulation section: 21 CFR 862.3150, Barbiturates test system 21 CFR 862.3200, Clinical Toxicology Calibrator 21 CFR 862.3180, Clinical Toxicology Control 2. Classification: Class II, (reagent, calibrator) Class I, reserved (control) 3. Product code: DIS, DLJ and DIF 4. Panel: Toxicology (91) 1 H. Intended Use: 1. Intended use(s): See Indications for use. 2. Indication(s) for use: VITROS Chemistry Products BARB Reagent: For in vitro diagnostic use only. VITROS Chemistry Products BARB Reagent is used on VITROS 5,1 FS Chemistry Systems for the semi- quantitative or qualitative determination of barbiturates (BARB) in human urine using a cutoff of 200 ng/mL or 300 ng/mL. Measurements obtained with the VITROS BARB method are used in the diagnosis and treatment of barbiturates use or overdose. The VITROS Chemistry Products BARB assay is intended for use by professional laboratory personnel. It provides only a preliminary test result. A more specific alternative chemical method must be used to confirm a result with this assay. Gas Chromatograpy/Mass Spectrometry (GC/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be applied to any drug-of-abuse test result, particularly when evaluating a preliminary positive result. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

IJBCP International Journal of Basic & Clinical Pharmacology Role Of
Print ISSN: 2319-2003 | Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology doi: 10.5455/2319-2003.ijbcp20131022 Research Article Role of piracetam on cognitive function in epilepsy and with antiepileptics in rats Siddharth R. Chaudhari1, Priti P. Dhande2*, Vijaya A. Pandit2 1Bristol-Myers Squibb India Pvt. ABSTRACT Ltd, Mumbai-13, Maharashtra, Background: To study extent of cognitive impairment by epilepsy & India 2Department of Pharmacology, antiepileptic treatment and evaluate the role of piracetam on it. Bharati Vidyapeeth (DU) Methods: 48 animals were divided into 6 groups: I-Control, II- Topiramate, III- Medical College, Pune- 43, Topiramate+Piracetam, IV-Valproate, V-Valproate+Piracetam, VI-Piracetam. Maharashtra, India Baseline cognitive functions were measured using Cook’s pole climbing apparatus (CPCA) and Elevated plus maze (EPM). In CPCA, on completion of Received: 10 August 2013 training, number of avoidances (NOA) out of 10 trials were noted while in Accepted: 18 August 2013 EPM, transfer latency (TL) was measured. Kindling was induced by 30mg/kg Pentylenetetrazol (PTZ), i.p. to all groups (except Group I) on alternate days till *Correspondence to: seizures developed. Groups were treated with respective drugs orally for 21 days and cognitive functions measured again. Dr. Priti P. Dhande, Email: [email protected] Results: Significant decrease in NOA & increase in TL was observed after PTZ kindling. Topiramate further significantly impaired NOA and TL whereas © 2013 Chaudhari SR et al. This Valproate significantly reduced NOA in CPCA but increase in TL was not is an open-access article significant. Treatment with Piracetam significantly increased Topiramate, Valproate and PTZ kindling induced decrease in NOA as also significantly distributed under the terms of the Creative Commons reduced Topiramate and PTZ kindling induced increase in TL. -

BULGARIA New Development, Trends and In-Depth Information on Selected Issues
Focal Point Logo 2013 NATIONAL REPORT (2012 data) TO THE EMCDDA by the Reitox National Focal Point BULGARIA New Development, Trends and in-depth information on selected issues REITOX Part A: New Developments and Trends 1. Drug policy: legislation, strategies and economic analysis 2. Drug use in the general population and specific targeted-groups 3. Prevention 4. Problem Drug Use 5. Drug-related treatment: treatment demand and treatment availability 6. Health correlates and consequences 7. Responses to Health Correlates and Consequences 8. Social correlates and social reintegration 9. Drug-related crime, prevention of drug related crime and prison 10. Drug Markets 2 1. Drug policy: legislation, strategies and economic analysis Within the framework of this section the following main topics will be reviewed: Legislative framework; National action plan, strategy, evaluation and coordination; Economic analysis; Legislative framework Acts, regulations, directives or guidelines in the sphere of drug addictions and drugs (supply and demand) In 2012 a total of nine amendments of the legislative regulation of the Republic of Bulgaria were adopted, including the adoption of two regulations and of seven amendments of the acts and legal regulations in the sphere of addictions. 1. On 20.06.2012 Regulation № 2 was adopted of the terms and conditions of implementing programmes for treatment with agonists and agonist-antagonists of individuals dependent on opioids. 1 By virtue of this regulation the following items are laid down: The terms and conditions for issuing an authorization for the implementation of programmes for treatment with agonists and agonist-antagonists of individuals dependent on opioids. The requirements for the individuals who can lead programmes and the requirements for the healthcare facilities where the programmes can be implemented. -

Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES REVIEW ARTICLE Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease Cheryl Waters ABSTRACT: Extensive recent literature on drugs used to enhance cognitive functioning, reflects the growing social problem of dementia. Many clinical trials have been undertaken with variable success. In most cases the disorder stud ied has been Alzheimer's disease. The pharmacological approach has been designed to rectify the presumed patho physiological processes characteristic of the condition. Agents tested include cerebral vasodilators, cerebral metabolic enhancers, nootropics, psychostimulants, neuropeptides and neurotransmitters with a special emphasis on drugs used to enhance cholinergic function. Ethical and practical issues concerning clinical drug trials in dementia will be discussed. RESUME: Stimulation cognitive medicamenteuse: etat de la question dans le traitement de la maladie d'Alzheimer La multiplicity des publications recentes sur les medicaments utilises pour stimuler le fonctionnement cognitif est le reflet du probl&me social sans cesse croissant de la d6mence. Plusieurs essais cliniques ont ete tentes avec des resultats variables. Dans la plupart des cas, la maladie etudiee etait la maladie d'Alzheimer. L'approche pharmacologique a ete con^ue pour corriger les processus physiopathologiques caracteristiques de la maladie. Les agents etudies incluent des vasodilatateurs cerebraux, des stimulants metaboliques cerebraux, des agents nootropes, des agents neurotropes, -

(12) Patent Application Publication (10) Pub. No.: US 2011/0263526 A1 SATYAM (43) Pub
US 20110263526A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0263526 A1 SATYAM (43) Pub. Date: Oct. 27, 2011 (54) NITRICOXIDE RELEASING PRODRUGS OF CD7C 69/96 (2006.01) THERAPEUTICAGENTS C07C 319/22 (2006.01) CD7C 68/02 (2006.01) (75) Inventor: Apparao SATYAM, Mumbai (IN) A6IP 29/00 (2006.01) A6IP 9/00 (2006.01) (73) Assignee: PIRAMAL LIFE SCIENCES A6IP37/08 (2006.01) LIMITED, Mumbai (IN) A6IP35/00 (2006.01) A6IP 25/24 2006.O1 (21) Appl. No.: 13/092.245 A6IP 25/08 308: A6IP3L/04 2006.O1 (22) Filed: Apr. 22, 2011 A6IP3L/2 308: Related U.S. Application Data 39t. O 308: (60) Provisional application No. 61/327,175, filed on Apr. A6IP3/10 (2006.01) 23, 2010. A6IPL/04 (2006.01) A6IP39/06 (2006.01) Publication Classification A6IP3/02 (2006.01) (51) Int. Cl. A6IP 9/06 (2006.01) A 6LX 3L/7072 (2006.01) 39t. W 308: A6 IK3I/58 (2006.01) A6IP II/08 (2006.015 A6 IK3I/55 (2006.01) A63/62 (2006.015 A6 IK 3/495 (2006.01) A6 IK 3/4439 (2006.01) (52) U.S. Cl. ........... 514/50: 514/166; 514/172: 514/217; A6 IK 3L/455 (2006.01) 514/255.04: 514/338; 514/356; 514/412; A6 IK 3/403 (2006.01) 514/420; 514/423: 514/510,536/28.53:540/67; A6 IK 3/404 (2006.01) 540/591; 544/396; 546/273.7: 546/318: 548/452: A6 IK 3/40 (2006.01) 548/500: 548/537; 549/464; 558/275 A6 IK3I/265 (2006.01) C7H 9/06 (2006.01) (57) ABSTRACT CO7I 71/00 (2006.01) CO7D 22.3/26 (2006.01) The present invention relates to nitric oxide releasing pro C07D 295/14 (2006.01) drugs of known drugs or therapeutic agents which are repre CO7D 40/12 (2006.01) sented herein as compounds of formula (I) wherein the drugs CO7D 213/80 (2006.01) or therapeutic agents contain one or more functional groups C07D 209/52 (2006.01) independently selected from a carboxylic acid, an amino, a CO7D 209/26 (2006.01) hydroxyl and a sulfhydryl group. -

Piracetam from Wikipedia, the Free Encyclopedia
Piracetam From Wikipedia, the free encyclopedia Systematic (IUPAC) name 2-oxo-1-pyrrolidineacetamide Clinical data Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Trade names Oikamid, and many others AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status POM (UK) Routes Oral and parenteral Pharmacokinetic data Bioavailability ~100% Half-life 4 - 5 hr Excretion Urinary Identifiers CAS number 7491-74-9 ATC code N06 BX03 PubChem CID 4843 ChemSpider 4677 UNII ZH516LNZ10 KEGG D01914 ChEMBL CHEMBL36715 Chemical data Formula C6H10N2O2 SMILES eMolecules & PubChem InChI Piracetam (sold under many brand names) is a nootropic drug. Piracetam's chemical name is 2-oxo-1- pyrrolidine acetamide; it shares the same 2-oxo-pyrrolidone base structure with 2-oxo-pyrrolidine carboxylic acid(pyroglutamate). Piracetam is a cyclic derivative of GABA. It is one of the group of racetams. Piracetam is prescribed by doctors for some conditions, mainly myoclonus,[1] but is used off-label for a much wider range of applications. Popular trade names for Piracetam in Europe are "Nootropil" and "Lucetam", among many others. In South America, it is made by Laboratorios Farma S.A. and sold under the brand name of Breinox in Venezuela and Ecuador. Contents • 1 Effects • 2 Mechanisms of action • 3 History • 4 Approval and usage • 4.1 Aging • 4.2 Alcoholism • 4.3 Alzheimer's and senile dementia • 4.4 Clotting, coagulation, vasospastic disorders • 4.5 Depression and anxiety • 4.6 Stroke, ischemia and symptoms • 4.7 Dyspraxia and dysgraphia • 4.8 Schizophrenia • 4.9 Preventive for breath-holding spells • 4.10 Closed craniocerebral trauma • 5 Dosage • 6 Side effects • 7 Availability • 8 Notes • 9 See also • 10 References • 11 External links Effects There is very little data on piracetam's effect on healthy people, with most studies focusing on people with seizures, dementia, concussions, or other neurological problems. -
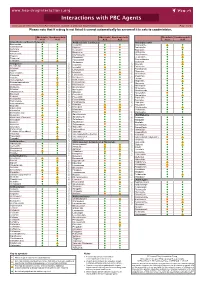
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created November 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Lymecycline Fluvoxamine Celecoxib Imipramine Meropenem Codeine distribution. for Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone distribution. for only. Not use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Ibuprofen Trazodone Rifampicin Indometacin -

ECO Cup One Step Drug Test Forensic Insert
paranoia, hallucinations, and psychotic behavior. The effects of Amphetamines generally last 2-4 unconsciousness. hours following use, and the drug has a half-life of 4-24 hours in the body. About 30% of Cocaine is often self-administered by nasal inhalation, intravenous injection and free-base Amphetamines are excreted in the urine in unchanged form, with the remainder as hydroxylated smoking. It is excreted in the urine in a short time primarily as Benzoylecgonine. 1.2 and deaminated derivatives. Benzoylecgonine, a major metabolite of cocaine, has a longer biological half-life (5-8 hours) than 2 The ECO CUP™ One Step Drug Test yields a positive result when the concentration of Amphetamine cocaine (0.5-1.5 hours), and can generally be detected for 24-48 hours after cocaine exposure. in urine exceeds 1,000 ng/mL. This is the suggested screening cut-off for positive specimens The ECO CUP™ One Step Drug Test yields a positive result when the concentration of Benzoylecgonine set by the Substance Abuse and Mental Health Services Administration (SAMHSA, USA).3 in urine exceeds 300 ng/mL. This is the suggested screening cut-off for positive specimens set by One Step Drug Test the Substance Abuse and Mental Health Services Administration (SAMHSA, USA). 3 Package Insert for Multi Drug Screen Test Cup AMPHETAMINE (AMP 500) COCAINE (COC 150) This Instruction Sheet is for testing of any combination of the following drugs: See AMPHETAMINE (AMP 1000) for the summary. See COCAINE (COC 300) for the summary. AMP/BAR/BZO/BUP/COC/THC/MTD/mAMP/MDMA/MOR/OPI/OXY/PCP/PPX/TCA/EDDP/6-ACM/ETG The ECO CUP™ One Step Drug Test yields a positive result when the concentration of The ECO CUP™ One Step Drug Test yields a positive result when the concentration of Including Adulterant Tests (Specimen Validity Tests) for: Amphetamine in urine exceeds 500 ng/mL.