Optimized and Validated Flow-Injection Spectrophotometric Analysis of Topiramate, Piracetam and Levetiracetam in Pharmaceutical Formulations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOOTROPIL® Piracetam
NOOTROPIL® Piracetam QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 800 mg or 1200 mg of piracetam. Each ml of oral solution contains 200 mg of piracetam EXCIPIENTS NOOTROPIL 800 mg and 1200 mg film-coated tablet: Core: Macrogol 6000 - Colloidal anhydrous silica - Magnesium stearate - Sodium croscarmellose Film-coating: Hydroxypropylmethylcellulose - Titanium dioxide (E171) - Macrogol 400 - Macrogol 6000. NOOTROPIL 200 mg/ml oral solution: Glycerol (85%) - Saccharin sodium - Apricot flavour - Caramel flavour - Methyl parahydroxybenzoate - Propyl parahydroxybenzoate - Sodium acetate - Glacial acetic acid - Purified water. PHARMACEUTICAL FORM NOOTROPIL Tablet 800 and 1200 mg: white, oblong, film-coated tablet, with a bisect line, marked N/N on one side and plain on the other side NOOTROPIL Oral Solution 20%: clear colourless solution INDICATIONS 1. Studies carried out in the elderly suffering from loss of memory, vertigo, a lack of concentration or of alertness, changes of mood, a deterioration in behaviour and personal negligence, demonstrate an improvement in symptoms. These symptoms can also provide an early warning of the onset of pathological ageing such as Alzheimer’s Disease, an Alzheimer type of senile dementia, or the dementia produced by multiple cerebral infarcts. 2. NOOTROPIL is advocated in the treatment of sickle-cell vaso-occlusive crises. 3. Studies have shown some improvement in children with learning difficulties associated with the written word, particularly with textual understanding which cannot be explained by intellectual backwardness, inadequate education or by the family environment. The administration of NOOTROPIL does not replace other measures also well adapted to correct these learning difficulties, such as remedial teaching. DOSAGE AND ADMINISTRATION Oral formulations 1 NOOTROPIL should be administered orally, and may be taken with or without food. -

Aniracetam Reduces Glutamate Receptor Desensitization and Slows
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 10936-10940, December 1991 Neurobiology Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus (non-N-methyl-D-aspartate receptor/synapse) JEFFRY S. ISAACSON*t AND ROGER A. NICOLLtt *Physiology Graduate Program and the Departments of SPharmacology and tPhysiology, University of California, San Francisco, CA 94143-0450 Communicated by Floyd E. Bloom, September 16, 1991 ABSTRACT Aniracetam is a nootropic drug that has been and DL-2-amino-5-phosphonovaleric acid (50 ,uM) were shown to selectively enhance quisqualate receptor-mediated added to the medium to block y-aminobutyric acid type A responses inXenopus oocytes injected with brain mRNA and in (GABAA) receptors and NMDA receptors, respectively. In hippocampal pyramidal cells [Ito, I., Tanabe, S., Kohda, A. & the majority of experiments examining iontophoretic re- Sugiyama, H. (1990) J. Physiol. (London) 424, 533-544]. We sponses, tetrodotoxin (0.5-1 1uM) was included to block have used patch clamp recording techniques in hippocampal sodium-dependent action potentials. Currents were recorded slices to elucidate the mechanism for this selective action. We with an Axopatch 1B amplifier from neurons in the CA1 and find that aniracetam enhances glutamate-evoked currents in CA3 pyramidal cell layers and granule cell layer of the whole-cell recordings and, in outside-out patches, strongly dentate gyrus using the "blind" whole-cell recording tech- reduces glutamate receptor desensitization. In addition, nique (15, 16). Patch electrodes (tip diameter = 2 Ium) aniracetam selectively prolongs the time course and increases contained (in mM) either a CsF (110 CsF, 10 CsCl, 10 Hepes, the peak amplitude of fast synaptic currents. -

September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. Joe Shia
September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. ℅ Joe Shia Manager LSI International 504 E Diamond Ave., Suite I Gaithersburg, MD 20877 Re: K202567 Trade/Device Name: Wondfo T-Dip® Multi-Drug Urine Test Panel Wondfo T-Dip® Multi-Drug Urine Test Panel Rx Regulation Number: 21 CFR 862.3100 Regulation Name: Amphetamine test system Regulatory Class: Class II Product Code: NFT, NGL, PTH, NFV, NFY, PTG, NGG, LCM, QBF, QAW, NFW Dated: September 2, 2020 Received: September 4, 2020 Dear Joe Shia: We have reviewed your Section 510(k) premarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, subject to the general controls provisions of the Act. Although this letter refers to your product as a device, please be aware that some cleared products may instead be combination products. The 510(k) Premarket Notification Database located at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm identifies combination product submissions. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. Please note: CDRH does not evaluate information related to contract liability warranties. -

IJBCP International Journal of Basic & Clinical Pharmacology Role Of
Print ISSN: 2319-2003 | Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology doi: 10.5455/2319-2003.ijbcp20131022 Research Article Role of piracetam on cognitive function in epilepsy and with antiepileptics in rats Siddharth R. Chaudhari1, Priti P. Dhande2*, Vijaya A. Pandit2 1Bristol-Myers Squibb India Pvt. ABSTRACT Ltd, Mumbai-13, Maharashtra, Background: To study extent of cognitive impairment by epilepsy & India 2Department of Pharmacology, antiepileptic treatment and evaluate the role of piracetam on it. Bharati Vidyapeeth (DU) Methods: 48 animals were divided into 6 groups: I-Control, II- Topiramate, III- Medical College, Pune- 43, Topiramate+Piracetam, IV-Valproate, V-Valproate+Piracetam, VI-Piracetam. Maharashtra, India Baseline cognitive functions were measured using Cook’s pole climbing apparatus (CPCA) and Elevated plus maze (EPM). In CPCA, on completion of Received: 10 August 2013 training, number of avoidances (NOA) out of 10 trials were noted while in Accepted: 18 August 2013 EPM, transfer latency (TL) was measured. Kindling was induced by 30mg/kg Pentylenetetrazol (PTZ), i.p. to all groups (except Group I) on alternate days till *Correspondence to: seizures developed. Groups were treated with respective drugs orally for 21 days and cognitive functions measured again. Dr. Priti P. Dhande, Email: [email protected] Results: Significant decrease in NOA & increase in TL was observed after PTZ kindling. Topiramate further significantly impaired NOA and TL whereas © 2013 Chaudhari SR et al. This Valproate significantly reduced NOA in CPCA but increase in TL was not is an open-access article significant. Treatment with Piracetam significantly increased Topiramate, Valproate and PTZ kindling induced decrease in NOA as also significantly distributed under the terms of the Creative Commons reduced Topiramate and PTZ kindling induced increase in TL. -

BULGARIA New Development, Trends and In-Depth Information on Selected Issues
Focal Point Logo 2013 NATIONAL REPORT (2012 data) TO THE EMCDDA by the Reitox National Focal Point BULGARIA New Development, Trends and in-depth information on selected issues REITOX Part A: New Developments and Trends 1. Drug policy: legislation, strategies and economic analysis 2. Drug use in the general population and specific targeted-groups 3. Prevention 4. Problem Drug Use 5. Drug-related treatment: treatment demand and treatment availability 6. Health correlates and consequences 7. Responses to Health Correlates and Consequences 8. Social correlates and social reintegration 9. Drug-related crime, prevention of drug related crime and prison 10. Drug Markets 2 1. Drug policy: legislation, strategies and economic analysis Within the framework of this section the following main topics will be reviewed: Legislative framework; National action plan, strategy, evaluation and coordination; Economic analysis; Legislative framework Acts, regulations, directives or guidelines in the sphere of drug addictions and drugs (supply and demand) In 2012 a total of nine amendments of the legislative regulation of the Republic of Bulgaria were adopted, including the adoption of two regulations and of seven amendments of the acts and legal regulations in the sphere of addictions. 1. On 20.06.2012 Regulation № 2 was adopted of the terms and conditions of implementing programmes for treatment with agonists and agonist-antagonists of individuals dependent on opioids. 1 By virtue of this regulation the following items are laid down: The terms and conditions for issuing an authorization for the implementation of programmes for treatment with agonists and agonist-antagonists of individuals dependent on opioids. The requirements for the individuals who can lead programmes and the requirements for the healthcare facilities where the programmes can be implemented. -

Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES REVIEW ARTICLE Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease Cheryl Waters ABSTRACT: Extensive recent literature on drugs used to enhance cognitive functioning, reflects the growing social problem of dementia. Many clinical trials have been undertaken with variable success. In most cases the disorder stud ied has been Alzheimer's disease. The pharmacological approach has been designed to rectify the presumed patho physiological processes characteristic of the condition. Agents tested include cerebral vasodilators, cerebral metabolic enhancers, nootropics, psychostimulants, neuropeptides and neurotransmitters with a special emphasis on drugs used to enhance cholinergic function. Ethical and practical issues concerning clinical drug trials in dementia will be discussed. RESUME: Stimulation cognitive medicamenteuse: etat de la question dans le traitement de la maladie d'Alzheimer La multiplicity des publications recentes sur les medicaments utilises pour stimuler le fonctionnement cognitif est le reflet du probl&me social sans cesse croissant de la d6mence. Plusieurs essais cliniques ont ete tentes avec des resultats variables. Dans la plupart des cas, la maladie etudiee etait la maladie d'Alzheimer. L'approche pharmacologique a ete con^ue pour corriger les processus physiopathologiques caracteristiques de la maladie. Les agents etudies incluent des vasodilatateurs cerebraux, des stimulants metaboliques cerebraux, des agents nootropes, des agents neurotropes, -

Piracetam from Wikipedia, the Free Encyclopedia
Piracetam From Wikipedia, the free encyclopedia Systematic (IUPAC) name 2-oxo-1-pyrrolidineacetamide Clinical data Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Trade names Oikamid, and many others AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status POM (UK) Routes Oral and parenteral Pharmacokinetic data Bioavailability ~100% Half-life 4 - 5 hr Excretion Urinary Identifiers CAS number 7491-74-9 ATC code N06 BX03 PubChem CID 4843 ChemSpider 4677 UNII ZH516LNZ10 KEGG D01914 ChEMBL CHEMBL36715 Chemical data Formula C6H10N2O2 SMILES eMolecules & PubChem InChI Piracetam (sold under many brand names) is a nootropic drug. Piracetam's chemical name is 2-oxo-1- pyrrolidine acetamide; it shares the same 2-oxo-pyrrolidone base structure with 2-oxo-pyrrolidine carboxylic acid(pyroglutamate). Piracetam is a cyclic derivative of GABA. It is one of the group of racetams. Piracetam is prescribed by doctors for some conditions, mainly myoclonus,[1] but is used off-label for a much wider range of applications. Popular trade names for Piracetam in Europe are "Nootropil" and "Lucetam", among many others. In South America, it is made by Laboratorios Farma S.A. and sold under the brand name of Breinox in Venezuela and Ecuador. Contents • 1 Effects • 2 Mechanisms of action • 3 History • 4 Approval and usage • 4.1 Aging • 4.2 Alcoholism • 4.3 Alzheimer's and senile dementia • 4.4 Clotting, coagulation, vasospastic disorders • 4.5 Depression and anxiety • 4.6 Stroke, ischemia and symptoms • 4.7 Dyspraxia and dysgraphia • 4.8 Schizophrenia • 4.9 Preventive for breath-holding spells • 4.10 Closed craniocerebral trauma • 5 Dosage • 6 Side effects • 7 Availability • 8 Notes • 9 See also • 10 References • 11 External links Effects There is very little data on piracetam's effect on healthy people, with most studies focusing on people with seizures, dementia, concussions, or other neurological problems. -
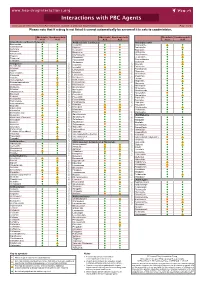
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created November 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Lymecycline Fluvoxamine Celecoxib Imipramine Meropenem Codeine distribution. for Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone distribution. for only. Not use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Ibuprofen Trazodone Rifampicin Indometacin -

Implications for the Dynamics of Long-Term Memory
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 2041-2045, March 1994 Psychology Delayed emergence of effects of memory-enhancing drugs: Implications for the dynamics of long-term memory CESARE MONDADORI, BASTIAN HENGERER, THOMAS DUCRET, AND JUERGEN BORKOWSKI Pharmaceutical Research Division, CIBA-Geigy Limited, Basle, CH-4002, Switzerland Communicated by L. Weiskrantz, November 22, 1993 (receivedfor review April 27, 1993) ABSTRACT Many theories of memory polate that pro- facilitation emerges, we hoped not only to help to character- cessing of information outlasts the earning situation and ize the substances themselves but also to shed light on the involves several different physiological substrates. If such dynamics of discrete phases of memory. The strategy of physiologically distinct mecanism or stages of memory do in examining time dependence of memory effects has a well- fact exist, they should be differentially affected by particular established history for substances that interfere with memory experimental manipulations. Accordingly, a selective improve- retention but has hardly been adopted in studies of memory ment ofthe processes underlying short-term memory should be enhancement. Even with interference treatments, reports of detectable only while the information is encoded in the short- delayed effects [as with cerebral electroshock, for example term mode, and a selective influence on long-term memory (14, 15)] and protein synthesis inhibition (e.g., see refs. should be detectable only from the moment when memory is 16-20) are relatively sparse. Moreover, some memory- based on the long-term trace. Our comparative study of the modulating substances have a wide range of unspecific side time course of the effects of the cholinergic agonist arecoline, effects that complicate interpretation-i.e., direct drug- the -aminobutyric acid type B receptor antagonist CGP induced behavioral effects can interfere with the behavioral 36742, the angiotensin-converting enzyme inhibitor captopril, manifestation of the memory effects at the time of retest. -

Pharmacology
STATE ESTABLISHMENT «DNIPROPETROVSK MEDICAL ACADEMY OF HEALTH MINISTRY OF UKRAINE» V.I. MAMCHUR, V.I. OPRYSHKO, А.А. NEFEDOV, A.E. LIEVYKH, E.V.KHOMIAK PHARMACOLOGY WORKBOOK FOR PRACTICAL CLASSES FOR FOREIGN STUDENTS STOMATOLOGY DEPARTMENT DNEPROPETROVSK - 2016 2 UDC: 378.180.6:61:615(075.5) Pharmacology. Workbook for practical classes for foreign stomatology students / V.Y. Mamchur, V.I. Opryshko, A.A. Nefedov. - Dnepropetrovsk, 2016. – 186 p. Reviewed by: N.I. Voloshchuk - MD, Professor of Pharmacology "Vinnitsa N.I. Pirogov National Medical University.‖ L.V. Savchenkova – Doctor of Medicine, Professor, Head of the Department of Clinical Pharmacology, State Establishment ―Lugansk state medical university‖ E.A. Podpletnyaya – Doctor of Pharmacy, Professor, Head of the Department of General and Clinical Pharmacy, State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ Approved and recommended for publication by the CMC of State Establishment ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ (protocol №3 from 25.12.2012). The educational tutorial contains materials for practical classes and final module control on Pharmacology. The tutorial was prepared to improve self-learning of Pharmacology and optimization of practical classes. It contains questions for self-study for practical classes and final module control, prescription tasks, pharmacological terms that students must know in a particular topic, medical forms of main drugs, multiple choice questions (tests) for self- control, basic and additional references. This tutorial is also a student workbook that provides the entire scope of student’s work during Pharmacology course according to the credit-modular system. The tutorial was drawn up in accordance with the working program on Pharmacology approved by CMC of SE ―Dnipropetrovsk medical academy of Health Ministry of Ukraine‖ on the basis of the standard program on Pharmacology for stomatology students of III - IV levels of accreditation in the specialties Stomatology – 7.110105, Kiev 2011. -

Pharmacotherapy for Stimulant Use Disorders: a Systematic Review
4 D epartment of Veterans Affairs Health Services Research & Development Service Evidence-based Synthesis Program Pharmacotherapy for Stimulant Use Disorders: A Systematic Review August 2018 Prepared for: Investigators: Department of Veterans Affairs Principal Investigator: Veterans Health Administration Brian Chan, MD, MPH Quality Enhancement Research Initiative Co-Investigators: Health Services Research & Development Service Karli Kondo, PhD, MA Washington, DC 20420 Chelsea Ayers, BA Prepared by: Michele Freeman, MPH Jessica Montgomery, MPH Evidence-based Synthesis Program (ESP) Robin Paynter, MLIS Portland VA Health Care System Devan Kansagara, MD, MCR Portland, OR Devan Kansagara, MD, MCR, Director 4 Pharmacotherapy for Stimulant Use Disorders Evidence-based Synthesis Program PREFACE The VA Evidence-based Synthesis Program (ESP) was established in 2007 to provide timely and accurate syntheses of targeted healthcare topics of particular importance to clinicians, managers, and policymakers as they work to improve the health and healthcare of Veterans. QUERI provides funding for 4 ESP Centers, and each Center has an active University affiliation. Center Directors are recognized leaders in the field of evidence synthesis with close ties to the AHRQ Evidence-based Practice Centers. The ESP is governed by a Steering Committee comprised of participants from VHA Policy, Program, and Operations Offices, VISN leadership, field-based investigators, and others as designated appropriate by QUERI/HSR&D. The ESP Centers generate evidence syntheses on important clinical practice topics. These reports help: · Develop clinical policies informed by evidence; · Implement effective services to improve patient outcomes and to support VA clinical practice guidelines and performance measures; and · Set the direction for future research to address gaps in clinical knowledge. -

Piracetam Potentiates Neuronal and Behavioral Effects of Ketamine
Research Results in Pharmacology 5(2): 49–55 UDC: 615.214.32+615.214.31-008.46 DOI 10.3897/rrpharmacology.5.35530 Research Article Piracetam potentiates neuronal and behavioral effects of ketamine Igor I. Abramets1, Yuriy V. Kuznetsov1, Dmitriy V. Evdokimov1, Tamara O. Zayka1 1 SEO HPE “Donetsk’s National Medical University named after M. Gorky” 16 Ilicha Ave., Donetsk 283003, Ukraine Corresponding author: Igor I. Abramets ([email protected]) Academic editor: Mikhail Korokin ♦ Received 17 April 2019 ♦ Accepted 5 June 2019 ♦ Published 27 June 2019 Citation: Abramets II, Kuznetsov YuV, Evdokimov DV, Zayka TO (2019) Piracetam potentiates neuronal and behavioral effects of ketamine. Research Results in Pharmacology 5(2): 49–55. https://doi.org/10.3897/rrpharmacology.5.35530 Abstract Introduction: Ketamine has a fast, but short-term antidepressant effect. To support the therapeutic effect, repeated administrations of the drug are needed, which causes cognitive disorders. The drugs with cerebroprotective action can potentially intensify the main and weaken the side effects of drugs. Materials and methods: The impact of ketamine (5 and 20 μM), piracetam (100 μM), and their combinations on the synaptic transmission was studied on hippocampal slices in the CA1 area of rat hippocampus by means of electrophys- iological methods. In behavioral experiments were aimed at studying an impact of the used drugs on the predictors which mark depressant behavior of rats: the duration of immobilization in a forced swimming test and preference for the consumption of sucrose solution (comparably with water). The behavioral experiments were performed on intact rats and rats with behavioral depression induced by chronic swimming stress.