BULGARIA New Development, Trends and In-Depth Information on Selected Issues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOOTROPIL® Piracetam
NOOTROPIL® Piracetam QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 800 mg or 1200 mg of piracetam. Each ml of oral solution contains 200 mg of piracetam EXCIPIENTS NOOTROPIL 800 mg and 1200 mg film-coated tablet: Core: Macrogol 6000 - Colloidal anhydrous silica - Magnesium stearate - Sodium croscarmellose Film-coating: Hydroxypropylmethylcellulose - Titanium dioxide (E171) - Macrogol 400 - Macrogol 6000. NOOTROPIL 200 mg/ml oral solution: Glycerol (85%) - Saccharin sodium - Apricot flavour - Caramel flavour - Methyl parahydroxybenzoate - Propyl parahydroxybenzoate - Sodium acetate - Glacial acetic acid - Purified water. PHARMACEUTICAL FORM NOOTROPIL Tablet 800 and 1200 mg: white, oblong, film-coated tablet, with a bisect line, marked N/N on one side and plain on the other side NOOTROPIL Oral Solution 20%: clear colourless solution INDICATIONS 1. Studies carried out in the elderly suffering from loss of memory, vertigo, a lack of concentration or of alertness, changes of mood, a deterioration in behaviour and personal negligence, demonstrate an improvement in symptoms. These symptoms can also provide an early warning of the onset of pathological ageing such as Alzheimer’s Disease, an Alzheimer type of senile dementia, or the dementia produced by multiple cerebral infarcts. 2. NOOTROPIL is advocated in the treatment of sickle-cell vaso-occlusive crises. 3. Studies have shown some improvement in children with learning difficulties associated with the written word, particularly with textual understanding which cannot be explained by intellectual backwardness, inadequate education or by the family environment. The administration of NOOTROPIL does not replace other measures also well adapted to correct these learning difficulties, such as remedial teaching. DOSAGE AND ADMINISTRATION Oral formulations 1 NOOTROPIL should be administered orally, and may be taken with or without food. -

St. St. Cyril and Methodius National Library
НАРОДНА БИБЛИОТЕКА “СВ . СВ . КИРИЛ И МЕТОДИЙ ” ST. ST. CYRIL AND METHODIUS NATIONAL LIBRARY бул . “ Васил Левски ” 88, 1037 София , тел . 00359 (2) 988 28 11, факс 00359 (2) 843 54 95 88, Vasil Levski blvd., 1037 Sofia, Bulgaria, phone 00359 (2) 988 28 11, fax 00359 (2) 843 54 95 http://www.nationallibrary.bg; e-mail: [email protected] Director: Prof. Boryana Hristova e-mail: [email protected] ANNUAL REPORT 2003 To Conference of the European National Librarians (CENL) 1. 1. Management of the library: 2003 was jubilee for the National Library of Bulgaria. All efforts of the staff were directed to celebrate with dignity the 125 Anniversary of the Library. The main goal of the celebrations was to put the National Library of Republic of Bulgaria and some of its most serious problems in front of the attention of the Bulgarian society. 2. Funding During 2003 the basis source of finance of the National Library was the subsidy allocated by the Ministry of Culture - 1 454 837 Bulgarian leva (746 070 Euro) – 85,94 % of the whole funding, including 118 410 leva (60 723 Euro) special grant for purchasing new computers. The Library’s own income is 257 479 leva (132 040 Euro) – 14,06 % of the whole funding. The own income was in proportion 67.60 income from provision of services, 26.46 from rents, 5.99 from sponsorship and 0.24 others. The National Library received 15 433 leva (7 914 Euro) sponsorship by private institutions and donations from individuals to purchase valuable collections, and to support publishing, exhibitions and other cultural events. -

Aniracetam Reduces Glutamate Receptor Desensitization and Slows
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 10936-10940, December 1991 Neurobiology Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus (non-N-methyl-D-aspartate receptor/synapse) JEFFRY S. ISAACSON*t AND ROGER A. NICOLLtt *Physiology Graduate Program and the Departments of SPharmacology and tPhysiology, University of California, San Francisco, CA 94143-0450 Communicated by Floyd E. Bloom, September 16, 1991 ABSTRACT Aniracetam is a nootropic drug that has been and DL-2-amino-5-phosphonovaleric acid (50 ,uM) were shown to selectively enhance quisqualate receptor-mediated added to the medium to block y-aminobutyric acid type A responses inXenopus oocytes injected with brain mRNA and in (GABAA) receptors and NMDA receptors, respectively. In hippocampal pyramidal cells [Ito, I., Tanabe, S., Kohda, A. & the majority of experiments examining iontophoretic re- Sugiyama, H. (1990) J. Physiol. (London) 424, 533-544]. We sponses, tetrodotoxin (0.5-1 1uM) was included to block have used patch clamp recording techniques in hippocampal sodium-dependent action potentials. Currents were recorded slices to elucidate the mechanism for this selective action. We with an Axopatch 1B amplifier from neurons in the CA1 and find that aniracetam enhances glutamate-evoked currents in CA3 pyramidal cell layers and granule cell layer of the whole-cell recordings and, in outside-out patches, strongly dentate gyrus using the "blind" whole-cell recording tech- reduces glutamate receptor desensitization. In addition, nique (15, 16). Patch electrodes (tip diameter = 2 Ium) aniracetam selectively prolongs the time course and increases contained (in mM) either a CsF (110 CsF, 10 CsCl, 10 Hepes, the peak amplitude of fast synaptic currents. -

1Daskalov R Tchavdar M Ed En
Entangled Histories of the Balkans Balkan Studies Library Editor-in-Chief Zoran Milutinović, University College London Editorial Board Gordon N. Bardos, Columbia University Alex Drace-Francis, University of Amsterdam Jasna Dragović-Soso, Goldsmiths, University of London Christian Voss, Humboldt University, Berlin Advisory Board Marie-Janine Calic, University of Munich Lenard J. Cohen, Simon Fraser University Radmila Gorup, Columbia University Robert M. Hayden, University of Pittsburgh Robert Hodel, Hamburg University Anna Krasteva, New Bulgarian University Galin Tihanov, Queen Mary, University of London Maria Todorova, University of Illinois Andrew Wachtel, Northwestern University VOLUME 9 The titles published in this series are listed at brill.com/bsl Entangled Histories of the Balkans Volume One: National Ideologies and Language Policies Edited by Roumen Daskalov and Tchavdar Marinov LEIDEN • BOSTON 2013 Cover Illustration: Top left: Krste Misirkov (1874–1926), philologist and publicist, founder of Macedo- nian national ideology and the Macedonian standard language. Photographer unknown. Top right: Rigas Feraios (1757–1798), Greek political thinker and revolutionary, ideologist of the Greek Enlightenment. Portrait by Andreas Kriezis (1816–1880), Benaki Museum, Athens. Bottom left: Vuk Karadžić (1787–1864), philologist, ethnographer and linguist, reformer of the Serbian language and founder of Serbo-Croatian. 1865, lithography by Josef Kriehuber. Bottom right: Şemseddin Sami Frashëri (1850–1904), Albanian writer and scholar, ideologist of Albanian and of modern Turkish nationalism, with his wife Emine. Photo around 1900, photo- grapher unknown. Library of Congress Cataloging-in-Publication Data Entangled histories of the Balkans / edited by Roumen Daskalov and Tchavdar Marinov. pages cm — (Balkan studies library ; Volume 9) Includes bibliographical references and index. -

September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. Joe Shia
September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. ℅ Joe Shia Manager LSI International 504 E Diamond Ave., Suite I Gaithersburg, MD 20877 Re: K202567 Trade/Device Name: Wondfo T-Dip® Multi-Drug Urine Test Panel Wondfo T-Dip® Multi-Drug Urine Test Panel Rx Regulation Number: 21 CFR 862.3100 Regulation Name: Amphetamine test system Regulatory Class: Class II Product Code: NFT, NGL, PTH, NFV, NFY, PTG, NGG, LCM, QBF, QAW, NFW Dated: September 2, 2020 Received: September 4, 2020 Dear Joe Shia: We have reviewed your Section 510(k) premarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, subject to the general controls provisions of the Act. Although this letter refers to your product as a device, please be aware that some cleared products may instead be combination products. The 510(k) Premarket Notification Database located at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm identifies combination product submissions. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. Please note: CDRH does not evaluate information related to contract liability warranties. -

IJBCP International Journal of Basic & Clinical Pharmacology Role Of
Print ISSN: 2319-2003 | Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology doi: 10.5455/2319-2003.ijbcp20131022 Research Article Role of piracetam on cognitive function in epilepsy and with antiepileptics in rats Siddharth R. Chaudhari1, Priti P. Dhande2*, Vijaya A. Pandit2 1Bristol-Myers Squibb India Pvt. ABSTRACT Ltd, Mumbai-13, Maharashtra, Background: To study extent of cognitive impairment by epilepsy & India 2Department of Pharmacology, antiepileptic treatment and evaluate the role of piracetam on it. Bharati Vidyapeeth (DU) Methods: 48 animals were divided into 6 groups: I-Control, II- Topiramate, III- Medical College, Pune- 43, Topiramate+Piracetam, IV-Valproate, V-Valproate+Piracetam, VI-Piracetam. Maharashtra, India Baseline cognitive functions were measured using Cook’s pole climbing apparatus (CPCA) and Elevated plus maze (EPM). In CPCA, on completion of Received: 10 August 2013 training, number of avoidances (NOA) out of 10 trials were noted while in Accepted: 18 August 2013 EPM, transfer latency (TL) was measured. Kindling was induced by 30mg/kg Pentylenetetrazol (PTZ), i.p. to all groups (except Group I) on alternate days till *Correspondence to: seizures developed. Groups were treated with respective drugs orally for 21 days and cognitive functions measured again. Dr. Priti P. Dhande, Email: [email protected] Results: Significant decrease in NOA & increase in TL was observed after PTZ kindling. Topiramate further significantly impaired NOA and TL whereas © 2013 Chaudhari SR et al. This Valproate significantly reduced NOA in CPCA but increase in TL was not is an open-access article significant. Treatment with Piracetam significantly increased Topiramate, Valproate and PTZ kindling induced decrease in NOA as also significantly distributed under the terms of the Creative Commons reduced Topiramate and PTZ kindling induced increase in TL. -

History of Modern Bulgarian Literature
The History ol , v:i IL Illlllf iM %.m:.:A Iiiil,;l|iBif| M283h UNIVERSITY OF FLORIDA LIBRARIES COLLEGE LIBRARY Digitized by the Internet Archive in 2012 with funding from LYRASIS Members and Sloan Foundation http://archive.org/details/historyofmodernbOOmann Modern Bulgarian Literature The History of Modern Bulgarian Literature by CLARENCE A. MANNING and ROMAN SMAL-STOCKI BOOKMAN ASSOCIATES :: New York Copyright © 1960 by Bookman Associates Library of Congress Catalog Card Number: 60-8549 MANUFACTURED IN THE UNITED STATES OF AMERICA BY UNITED PRINTING SERVICES, INC. NEW HAVEN, CONN. Foreword This outline of modern Bulgarian literature is the result of an exchange of memories of Bulgaria between the authors some years ago in New York. We both have visited Bulgaria many times, we have had many personal friends among its scholars and statesmen, and we feel a deep sympathy for the tragic plight of this long-suffering Slavic nation with its industrious and hard-working people. We both feel also that it is an injustice to Bulgaria and a loss to American Slavic scholarship that, in spite of the importance of Bulgaria for the Slavic world, so little attention is paid to the country's cultural contributions. This is the more deplorable for American influence in Bulgaria was great, even before World War I. Many Bulgarians were educated in Robert Col- lege in Constantinople and after World War I in the American College in Sofia, one of the institutions supported by the Near East Foundation. Many Bulgarian professors have visited the United States in happier times. So it seems unfair that Ameri- cans and American universities have ignored so completely the development of the Bulgarian genius and culture during the past century. -

Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease
LE JOURNAL CANADIEN DES SCIENCES NEUROLOGIQUES REVIEW ARTICLE Cognitive Enhancing Agents: Current Status in the Treatment of Alzheimer's Disease Cheryl Waters ABSTRACT: Extensive recent literature on drugs used to enhance cognitive functioning, reflects the growing social problem of dementia. Many clinical trials have been undertaken with variable success. In most cases the disorder stud ied has been Alzheimer's disease. The pharmacological approach has been designed to rectify the presumed patho physiological processes characteristic of the condition. Agents tested include cerebral vasodilators, cerebral metabolic enhancers, nootropics, psychostimulants, neuropeptides and neurotransmitters with a special emphasis on drugs used to enhance cholinergic function. Ethical and practical issues concerning clinical drug trials in dementia will be discussed. RESUME: Stimulation cognitive medicamenteuse: etat de la question dans le traitement de la maladie d'Alzheimer La multiplicity des publications recentes sur les medicaments utilises pour stimuler le fonctionnement cognitif est le reflet du probl&me social sans cesse croissant de la d6mence. Plusieurs essais cliniques ont ete tentes avec des resultats variables. Dans la plupart des cas, la maladie etudiee etait la maladie d'Alzheimer. L'approche pharmacologique a ete con^ue pour corriger les processus physiopathologiques caracteristiques de la maladie. Les agents etudies incluent des vasodilatateurs cerebraux, des stimulants metaboliques cerebraux, des agents nootropes, des agents neurotropes, -

Piracetam from Wikipedia, the Free Encyclopedia
Piracetam From Wikipedia, the free encyclopedia Systematic (IUPAC) name 2-oxo-1-pyrrolidineacetamide Clinical data Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Trade names Oikamid, and many others AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status POM (UK) Routes Oral and parenteral Pharmacokinetic data Bioavailability ~100% Half-life 4 - 5 hr Excretion Urinary Identifiers CAS number 7491-74-9 ATC code N06 BX03 PubChem CID 4843 ChemSpider 4677 UNII ZH516LNZ10 KEGG D01914 ChEMBL CHEMBL36715 Chemical data Formula C6H10N2O2 SMILES eMolecules & PubChem InChI Piracetam (sold under many brand names) is a nootropic drug. Piracetam's chemical name is 2-oxo-1- pyrrolidine acetamide; it shares the same 2-oxo-pyrrolidone base structure with 2-oxo-pyrrolidine carboxylic acid(pyroglutamate). Piracetam is a cyclic derivative of GABA. It is one of the group of racetams. Piracetam is prescribed by doctors for some conditions, mainly myoclonus,[1] but is used off-label for a much wider range of applications. Popular trade names for Piracetam in Europe are "Nootropil" and "Lucetam", among many others. In South America, it is made by Laboratorios Farma S.A. and sold under the brand name of Breinox in Venezuela and Ecuador. Contents • 1 Effects • 2 Mechanisms of action • 3 History • 4 Approval and usage • 4.1 Aging • 4.2 Alcoholism • 4.3 Alzheimer's and senile dementia • 4.4 Clotting, coagulation, vasospastic disorders • 4.5 Depression and anxiety • 4.6 Stroke, ischemia and symptoms • 4.7 Dyspraxia and dysgraphia • 4.8 Schizophrenia • 4.9 Preventive for breath-holding spells • 4.10 Closed craniocerebral trauma • 5 Dosage • 6 Side effects • 7 Availability • 8 Notes • 9 See also • 10 References • 11 External links Effects There is very little data on piracetam's effect on healthy people, with most studies focusing on people with seizures, dementia, concussions, or other neurological problems. -
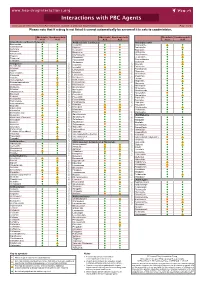
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created November 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Lymecycline Fluvoxamine Celecoxib Imipramine Meropenem Codeine distribution. for Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone distribution. for only. Not use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Ibuprofen Trazodone Rifampicin Indometacin -

National Theatre "Ivan Vazov"
National Theatre "Ivan Vazov" – the cultural heart of Sofia Slide 1: Introduction The Ivan Vazov National Theater is located in the center of Sofia (the capital of Bulgaria). In front of the building is one of the most visited parks in the capital. It's known as the City Garden. The theater, has been chosen to be located near the Tzar’s Palace – former Royal Palace (nowadays the National Art Gallery). Slide 2: For more than 100 years, the Ivan Vazov National Theater has been recognized not only as a stage in Bulgaria, but also as a living, working and large-scale theater, that is aware of the important mission as a national cultural institute. The history of the theater is rich, interesting, contrasting in its rises and crisis, but always firmly connected with the essential changes and directions in the development of national culture and art. Slide 3: History In modernizing Sofia at the end of the 19th century, there was long a talk about the need to build a special building for the state theater, which, as the national poet Ivan Vazov points out, would give better opportunities "for the future greatness of dramatic art in Bulgaria". In December 1898, with a decision by the National Assembly was created a special fund for the construction of the building. Slide 4: The theater was designed by the famous Viennese architects Ferdinand Felner and Herman Helmer, who have already made numerous theatrical buildings in Vienna, Zagreb, Prague and other European cities. The construction started in 1904 on the site of the obsolete “Foundation” wooden playhouse, where previously the first professional troupe "Tear and Laughter" played its performances, and at the end of 1906 the building was completed. -

Optimized and Validated Flow-Injection Spectrophotometric Analysis of Topiramate, Piracetam and Levetiracetam in Pharmaceutical Formulations
Acta Pharm. 61 (2011) 377–389 Original research paper DOI: 10.2478/v10007-011-0038-y Optimized and validated flow-injection spectrophotometric analysis of topiramate, piracetam and levetiracetam in pharmaceutical formulations GHADA M. HADAD1 Application of a sensitive and rapid flow injection analy- 1 RANDA A. ABDEL-SALAM sis (FIA) method for determination of topiramate, pira- SAMY EMARA2 cetam, and levetiracetam in pharmaceutical formulations 1 Pharmaceutical Analytical Chemistry has been investigated. The method is based on the reac- Department, Faculty of Pharmacy tion with ortho-phtalaldehyde and 2-mercaptoethanol in University of Suez Canal a basic buffer and measurement of absorbance at 295 nm Ismailia 41522, Egypt under flow conditions. Variables affecting the determina- tion such as sample injection volume, pH, ionic strength, 2 Pharmaceutical Analytical Chemistry reagent concentrations, flow rate of reagent and other FIA Department, Faculty of Pharmacy parameters were optimized to produce the most sensiti- Misr International University, Egypt ve and reproducible results using a quarter-fraction fac- torial design, for five factors at two levels. Also, the me- thod has been optimized and fully validated in terms of linearity and range, limit of detection and quantitation, precision, selectivity and accuracy. The method was suc- cessfully applied to the analysis of pharmaceutical prepa- rations. Keywords: topiramate, piracetam, levetiracetam, flow-in- Accepted October 31, 2011 jection analysis Flow injection analysis (FIA) has become a technique of increasing importance in pharmaceutical analysis because of its implicit simplicity, low cost and rapid approach. FIA is a continuous flow method in which a small plug of sample is injected into a flow- ing reagent stream.