Intended Use Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Programme & Abstracts
The 57th Annual Meeting of the International Association of Forensic Toxicologists. 2nd - 6th September 2019 BIRMINGHAM, UK The ICC Birmingham Broad Street, Birmingham B1 2EA Programme & Abstracts 1 Thank You to our Sponsors PlatinUm Gold Silver Bronze 2 3 Contents Welcome message 5 Committees 6 General information 7 iCC maps 8 exhibitors list 10 Exhibition Hall 11 Social Programme 14 opening Ceremony 15 Schedule 16 Oral Programme MONDAY 2 September 19 TUESDAY 3 September 21 THURSDAY 5 September 28 FRIDAY 6 September 35 vendor Seminars 42 Posters 46 oral abstracts 82 Poster abstracts 178 4 Welcome Message It is our great pleasure to welcome you to TIAFT Gala Dinner at the ICC on Friday evening. On the accompanying pages you will see a strong the UK for the 57th Annual Meeting of scientific agenda relevant to modern toxicology and we The International Association of Forensic thank all those who submitted an abstract and the Toxicologists Scientific Committees for making the scientific programme (TIAFT) between 2nd and 6th a success. Starting with a large Young Scientists September 2019. Symposium and Dr Yoo Memorial plenary lecture by Prof Tony Moffat on Monday, there are oral session topics in It has been decades since the Annual Meeting has taken Clinical & Post-Mortem Toxicology on Tuesday, place in the country where TIAFT was founded over 50 years Human Behaviour Toxicology & Drug-Facilitated Crime on ago. The meeting is supported by LTG (London Toxicology Thursday and Toxicology in Sport, New Innovations and Group) and the UKIAFT (UK & Ireland Association of Novel Research & Employment/Occupational Toxicology Forensic Toxicologists) and we thank all our exhibitors and on Friday. -
Cross Reaction Guide
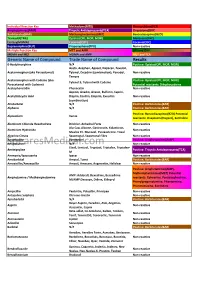
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

Oral Fluid Drug Test Package Insert
Marijuana (THC) 11-nor-Δ9-THC-9 COOH 4 MTD: Methadone is a synthetic analgesic drug originally used for the Oral Fluid Drug Test treatment of narcotic addiction. In addition to use as a narcotic agonist, methadone is being used more frequently as a pain management agent. The Marijuana (THC) Δ9-THC 50 Package Insert psychological effects induced by using methadone are analgesia, sedation, and respiratory depression. Based on the saliva/plasma ratio calculated over Package insert for testing of the following drugs: > 0.02 % Alcohol (ALC) Alcohol salivary pH ranges of 6.4-7.6 for therapeutic or recreational doses of Amphetamine,Cocaine,Marijuana,Methamphetamine,Opiate,Methadone, B.A.C methadone, a cut-off <50 ng/mL is suggested. Due to this recommendation, Phencyclidine,Oxycodone,Benzodiazepine,Buprenorphine,Barbiturates, the cut-off level of the methadone test was calibrated to 30 ng/mL. Cotinine,EDDP,MDMA,6-MAM,Propoxyphene ,Fentanyl,ETG and Alcohol Tramadol(TRA) 50 Tramadol PCP: Phencyclidine is a hallucinogen and, can be detected in oral fluid as a INTENDED USE & SUMMARY result of the exchange of the drug between the circulatory system and the oral cavity.5 Fentanyl(FEN) 10 The Oral Fluid Drug And Alcohol Test is intended for screening for the Norfentanyl presence of drugs and alcohol and their metabolites in oral fluid. For professional in vitro diagnostic use only. OXY: Oxycodone is a semi-synthetic opioid with a structural similarity to Ethyl codeine. The drug is manufactured by modifying thebaine, an alkaloid found in 150 The Oral Fluid Pipette Test is a lateral flow chromatographic immunoassay for Glucuronide(ETG) the opium poppy. -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied. -

Review Memorandum
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k062165 B. Purpose for Submission: New device C. Measurand: Barbiturates D. Type of Test: Qualitative and semi-quantitative enzyme immunoassay E. Applicant: Ortho-Clinical Diagnostics, Inc. F. Proprietary and Established Names: VITROS Chemistry Products BARB Reagent VITROS Chemistry Products Calibrator 26 VITROS Chemistry Products FS Calibrator 1 VITROS Chemistry Products DAT Performance Verifiers I, II, III, IV and V G. Regulatory Information: 1. Regulation section: 21 CFR 862.3150, Barbiturates test system 21 CFR 862.3200, Clinical Toxicology Calibrator 21 CFR 862.3180, Clinical Toxicology Control 2. Classification: Class II, (reagent, calibrator) Class I, reserved (control) 3. Product code: DIS, DLJ and DIF 4. Panel: Toxicology (91) 1 H. Intended Use: 1. Intended use(s): See Indications for use. 2. Indication(s) for use: VITROS Chemistry Products BARB Reagent: For in vitro diagnostic use only. VITROS Chemistry Products BARB Reagent is used on VITROS 5,1 FS Chemistry Systems for the semi- quantitative or qualitative determination of barbiturates (BARB) in human urine using a cutoff of 200 ng/mL or 300 ng/mL. Measurements obtained with the VITROS BARB method are used in the diagnosis and treatment of barbiturates use or overdose. The VITROS Chemistry Products BARB assay is intended for use by professional laboratory personnel. It provides only a preliminary test result. A more specific alternative chemical method must be used to confirm a result with this assay. Gas Chromatograpy/Mass Spectrometry (GC/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be applied to any drug-of-abuse test result, particularly when evaluating a preliminary positive result. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. Joe Shia
September 25, 2020 Guangzhou Wondfo Biotech Co., Ltd. ℅ Joe Shia Manager LSI International 504 E Diamond Ave., Suite I Gaithersburg, MD 20877 Re: K202567 Trade/Device Name: Wondfo T-Dip® Multi-Drug Urine Test Panel Wondfo T-Dip® Multi-Drug Urine Test Panel Rx Regulation Number: 21 CFR 862.3100 Regulation Name: Amphetamine test system Regulatory Class: Class II Product Code: NFT, NGL, PTH, NFV, NFY, PTG, NGG, LCM, QBF, QAW, NFW Dated: September 2, 2020 Received: September 4, 2020 Dear Joe Shia: We have reviewed your Section 510(k) premarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, subject to the general controls provisions of the Act. Although this letter refers to your product as a device, please be aware that some cleared products may instead be combination products. The 510(k) Premarket Notification Database located at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm identifies combination product submissions. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. Please note: CDRH does not evaluate information related to contract liability warranties. -

HANDBOOK of Medicinal Herbs SECOND EDITION
HANDBOOK OF Medicinal Herbs SECOND EDITION 1284_frame_FM Page 2 Thursday, May 23, 2002 10:53 AM HANDBOOK OF Medicinal Herbs SECOND EDITION James A. Duke with Mary Jo Bogenschutz-Godwin Judi duCellier Peggy-Ann K. Duke CRC PRESS Boca Raton London New York Washington, D.C. Peggy-Ann K. Duke has the copyright to all black and white line and color illustrations. The author would like to express thanks to Nature’s Herbs for the color slides presented in the book. Library of Congress Cataloging-in-Publication Data Duke, James A., 1929- Handbook of medicinal herbs / James A. Duke, with Mary Jo Bogenschutz-Godwin, Judi duCellier, Peggy-Ann K. Duke.-- 2nd ed. p. cm. Previously published: CRC handbook of medicinal herbs. Includes bibliographical references and index. ISBN 0-8493-1284-1 (alk. paper) 1. Medicinal plants. 2. Herbs. 3. Herbals. 4. Traditional medicine. 5. Material medica, Vegetable. I. Duke, James A., 1929- CRC handbook of medicinal herbs. II. Title. [DNLM: 1. Medicine, Herbal. 2. Plants, Medicinal.] QK99.A1 D83 2002 615′.321--dc21 2002017548 This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. -

(LSD) Test Dip Card (Urine) • Specimen Collection Container % Agreement 98.8% 99
frozen and stored below -20°C. Frozen specimens should be thawed and mixed before testing. GC/MS. The following results were tabulated: Method GC/MS MATERIALS Total Results Results Positive Negative Materials Provided LSD Rapid Positive 79 1 80 LSD • Test device • Desiccants • Package insert • Urine cups Test Dip card Negative 1 99 100 Materials Required But Not Provided Total Results 80 100 180 One Step Lysergic acid diethylamide (LSD) Test Dip card (Urine) • Specimen collection container % Agreement 98.8% 99. % 98.9% • Timer Package Insert DIRECTIONS FOR USE Analytical Sensitivity This Instruction Sheet is for testing of Lysergic acid diethylamide. Allow the test device, and urine specimen to come to room temperature [15-30°C (59-86°F)] prior to testing. A drug-free urine pool was spiked with LSD at the following concentrations: 0 ng/mL, -50%cutoff, -25%cutoff, cutoff, A rapid, one step test for the qualitative detection of Lysergic acid diethylamide and its metabolites in human urine. 1) Remove the test device from the foil pouch. +25%cutoff and +50%cutoff. The result demonstrates >99% accuracy at 50% above and 50% below the cut-off For forensic use only. 2) Remove the cap from the test device. Label the device with patient or control identifications. concentration. The data are summarized below: INTENDED USE 3) Immerse the absorbent tip into the urine sample for 10-15 seconds. Urine sample should not touch the plastic Lysergic acid diethylamide (LSD) Percent of Visual Result The One Step Lysergic acid diethylamide (LSD) Test Dip card (Urine) is a lateral flow chromatographic device. -

Third ESVAC Report
Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011 Third ESVAC report An agency of the European Union The mission of the European Medicines Agency is to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health. Legal role Guiding principles The European Medicines Agency is the European Union • We are strongly committed to public and animal (EU) body responsible for coordinating the existing health. scientific resources put at its disposal by Member States • We make independent recommendations based on for the evaluation, supervision and pharmacovigilance scientific evidence, using state-of-the-art knowledge of medicinal products. and expertise in our field. • We support research and innovation to stimulate the The Agency provides the Member States and the development of better medicines. institutions of the EU the best-possible scientific advice on any question relating to the evaluation of the quality, • We value the contribution of our partners and stake- safety and efficacy of medicinal products for human or holders to our work. veterinary use referred to it in accordance with the • We assure continual improvement of our processes provisions of EU legislation relating to medicinal prod- and procedures, in accordance with recognised quality ucts. standards. • We adhere to high standards of professional and Principal activities personal integrity. Working with the Member States and the European • We communicate in an open, transparent manner Commission as partners in a European medicines with all of our partners, stakeholders and colleagues. network, the European Medicines Agency: • We promote the well-being, motivation and ongoing professional development of every member of the • provides independent, science-based recommenda- Agency. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0263526 A1 SATYAM (43) Pub
US 20110263526A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0263526 A1 SATYAM (43) Pub. Date: Oct. 27, 2011 (54) NITRICOXIDE RELEASING PRODRUGS OF CD7C 69/96 (2006.01) THERAPEUTICAGENTS C07C 319/22 (2006.01) CD7C 68/02 (2006.01) (75) Inventor: Apparao SATYAM, Mumbai (IN) A6IP 29/00 (2006.01) A6IP 9/00 (2006.01) (73) Assignee: PIRAMAL LIFE SCIENCES A6IP37/08 (2006.01) LIMITED, Mumbai (IN) A6IP35/00 (2006.01) A6IP 25/24 2006.O1 (21) Appl. No.: 13/092.245 A6IP 25/08 308: A6IP3L/04 2006.O1 (22) Filed: Apr. 22, 2011 A6IP3L/2 308: Related U.S. Application Data 39t. O 308: (60) Provisional application No. 61/327,175, filed on Apr. A6IP3/10 (2006.01) 23, 2010. A6IPL/04 (2006.01) A6IP39/06 (2006.01) Publication Classification A6IP3/02 (2006.01) (51) Int. Cl. A6IP 9/06 (2006.01) A 6LX 3L/7072 (2006.01) 39t. W 308: A6 IK3I/58 (2006.01) A6IP II/08 (2006.015 A6 IK3I/55 (2006.01) A63/62 (2006.015 A6 IK 3/495 (2006.01) A6 IK 3/4439 (2006.01) (52) U.S. Cl. ........... 514/50: 514/166; 514/172: 514/217; A6 IK 3L/455 (2006.01) 514/255.04: 514/338; 514/356; 514/412; A6 IK 3/403 (2006.01) 514/420; 514/423: 514/510,536/28.53:540/67; A6 IK 3/404 (2006.01) 540/591; 544/396; 546/273.7: 546/318: 548/452: A6 IK 3/40 (2006.01) 548/500: 548/537; 549/464; 558/275 A6 IK3I/265 (2006.01) C7H 9/06 (2006.01) (57) ABSTRACT CO7I 71/00 (2006.01) CO7D 22.3/26 (2006.01) The present invention relates to nitric oxide releasing pro C07D 295/14 (2006.01) drugs of known drugs or therapeutic agents which are repre CO7D 40/12 (2006.01) sented herein as compounds of formula (I) wherein the drugs CO7D 213/80 (2006.01) or therapeutic agents contain one or more functional groups C07D 209/52 (2006.01) independently selected from a carboxylic acid, an amino, a CO7D 209/26 (2006.01) hydroxyl and a sulfhydryl group.