Programme & Abstracts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inclusion of People of Color in Psychedelic-Assisted Psychotherapy
Michaels et al. BMC Psychiatry (2018) 18:245 https://doi.org/10.1186/s12888-018-1824-6 RESEARCH ARTICLE Open Access Inclusion of people of color in psychedelic- assisted psychotherapy: a review of the literature Timothy I. Michaels1* , Jennifer Purdon1,2, Alexis Collins1 and Monnica T. Williams1,2 Abstract Background: Despite renewed interest in studying the safety and efficacy of psychedelic-assisted psychotherapy for the treatment of psychological disorders, the enrollment of racially diverse participants and the unique presentation of psychopathology in this population has not been a focus of this potentially ground-breaking area of research. In 1993, the United States National Institutes of Health issued a mandate that funded research must include participants of color and proposals must include methods for achieving diverse samples. Methods: A methodological search of psychedelic studies from 1993 to 2017 was conducted to evaluate ethnoracial differences in inclusion and effective methods of recruiting peopple of color. Results: Of the 18 studies that met full criteria (n = 282 participants), 82.3% of the participants were non-Hispanic White, 2.5% were African-American, 2.1% were of Latino origin, 1.8% were of Asian origin, 4.6% were of indigenous origin, 4.6% were of mixed race, 1.8% identified their race as “other,” and the ethnicity of 8.2% of participants was unknown. There were no significant differences in recruitment methodologies between those studies that had higher (> 20%) rates of inclusion. Conclusions: As minorities are greatly underrepresented in psychedelic medicine studies, reported treatment outcomes may not generalize to all ethnic and cultural groups. -

American Civil Liberties Union
WASHINGTON LEGISLATIVE OFFICE March 19, 2012 Honorable Patti B. Saris, Chair United States Sentencing Commission One Columbus Circle, N.E. Suite 2-500, South Lobby Washington, D.C. 2002-8002 Re: ACLU Comments on Proposed Amendments to Sentencing Guidelines, Policy Statements, and Commentary due on March 19, 2012 AMERICAN CIVIL LIBERTIES UNION WASHINGTON Dear Judge Saris: LEGISLATIVE OFFICE 915 15th STREET, NW, 6 TH FL WASHINGTON, DC 20005 With this letter the American Civil Liberties Union (“ACLU”) provides T/202.544.1681 commentary on the Amendments to the U.S. Sentencing Guidelines F/202.546.0738 WWW.ACLU.ORG (“Guidelines”) proposed by the Commission on January 19, 2012. The American Civil Liberties Union is a non-partisan organization with more than LAURA W. MURPHY DIRECTOR half a million members, countless additional activists and supporters, and 53 NATIONAL OFFICE affiliates nationwide dedicated to the principles of liberty, equality, and justice 125 BROAD STREET, 18 TH FL. embodied in our Constitution and our civil rights laws. NEW YORK, NY 10004-2400 T/212.549.2500 These comments address four issues that the Commission has asked for OFFICERS AND DIRECTORS SUSAN N. HERMAN public comment on by March 19, 2012. First, the ACLU encourages the PRESIDENT Commission to reject the adoption of the 500:1 MDMA marijuana equivalency ANTHONY D. ROMERO ratio for N-Benzylpiperazine, also known as BZP, (BZP) and make substantial EXECUTIVE DIRECTOR downward revisions to the MDMA marijuana equivalency ratio. Also, we urge ROBERT REMAR the Commission to respect the principles of proportionality and due process in TREASURER deciding how and whether to amend the Guideline for unlawfully entering or remaining in the country. -

Substance Abuse: the Pharmacy Educator’S Role in Prevention and Recovery
Substance Abuse: The Pharmacy Educator’s Role in Prevention and Recovery Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease i Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease1,2 BACKGROUND OF THE CURRICULUM DEVELOPMENT PROJECT In 1988, the AACP Special Interest Group (SIG) on Pharmacy Student and Faculty Impairment (renamed Substance Abuse Education and Assistance) undertook the development of curricular guidelines for colleges/schools of pharmacy to facilitate the growth of educational opportunities for student pharmacists. These Curricular Guidelines for Pharmacy Education: Substance Abuse and Addictive Disease were published in 1991 (AJPE. 55:311-16. Winter 1991.) One of the charges of the Special Committee on Substance Abuse and Pharmacy Education was to review and revise the 1991 curricular guidelines. Overall, the didactic and experiential components in the suggested curriculum should prepare the student pharmacist to competently problem-solve issues concerning alcohol and other drug abuse and addictive diseases affecting patients, families, colleagues, themselves, and society. The guidelines provide ten educational goals, while describing four major content areas including: psychosocial aspects of alcohol and other drug use; pharmacology and toxicology of abused substances; identification, intervention, and treatment of people with addictive diseases; and legal/ethical issues. The required curriculum suggested by these guidelines addresses the 1 These guidelines were revised by the AACP Special Committee on Substance Abuse and Pharmacy Education. Members drafting the revised guidelines were Edward M. DeSimone (Creighton University), Julie C. Kissack (Harding University), David M. Scott (North Dakota State University), and Brandon J. Patterson (University of Iowa). Other Committee members were Paul W. Jungnickel, Chair (Auburn University), Lisa A. -

Initial Considerations for the Creation of an Inter-Regional Industrial Hemp Value Chain Between Malawi and South Africa
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Lowitt, Sandy Working Paper Initial considerations for the creation of an inter- regional industrial hemp value chain between Malawi and South Africa WIDER Working Paper, No. 2020/23 Provided in Cooperation with: United Nations University (UNU), World Institute for Development Economics Research (WIDER) Suggested Citation: Lowitt, Sandy (2020) : Initial considerations for the creation of an inter- regional industrial hemp value chain between Malawi and South Africa, WIDER Working Paper, No. 2020/23, ISBN 978-92-9256-780-4, The United Nations University World Institute for Development Economics Research (UNU-WIDER), Helsinki, http://dx.doi.org/10.35188/UNU-WIDER/2020/780-4 This Version is available at: http://hdl.handle.net/10419/229247 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

Drug Education Student Booklet
WHAT YOU NEED TO KNOW STUDENT VERSION CONTENTS What is a drug? 3 How many young people use drugs and alcohol? 4 Drug and alcohol use and the law 6 Making choices 7 How to help a friend who has taken a drug 10 DRS-ABCD: Basic life support flow chart 13 Putting someone in the recovery position 14 How to help a friend or family member with their drug or alcohol use 15 Drugs A-Z Alcohol 17 Benzodiazepines 18 Cannabis 19 Cocaine 20 GHB 21 Hallucinogens 22 Heroin 23 Inhalants 24 Ketamine 25 MDMA/Ecstasy 26 Methamphetamine 27 New psychoactive substances 28 Synthetic cannabinoids 29 Performance and image-enhancing drugs 30 Tobacco & e-cigarettes 31 Polydrug use 32 Notes 33 Glossary 36 More information and sources of help 37 2 WHAT IS A DRUG? Drugs (including alcohol) are substances that affect the way the body functions when they are used. If a drug is illegal it means that it is forbidden by law. Different drugs have different effects on people, and different factors can impact on the experience of drug use. These include: ● the drug itself (e.g. the pharmacological properties of the substance being taken); ● the individual taking the drug (e.g. age, sex, physical and mental health of a person); ● the environment (the setting where the drug is being used, which can be legal, cultural or situational). Drinking two or three beers might be relatively low risk for a healthy adult but the risk of harm increases if they drink on an empty stomach, try to drive a car after drinking, or have a pre- existing health problem. -

Ecstasy Or Molly, Is a Synthetic Psychoactive Drug
DRUG ENFORCEMENT ADMINISTRATION THE FACTS ABOUT MDMA Ecstasy &Molly DEA PHOTO What is it? MDMA, (3,4-methylenedioxy- methamphetamine), known as Ecstasy or Molly, is a synthetic psychoactive drug. Ecstasy is the pill form of MDMA. Molly is the slang for “molecular” that refers to the powder or crystal form of MDMA. Molly is often mixed with other drugs and substances and is not pure MDMA or safe to use. How is it used? MDMA or Ecstasy is taken orally in pill or tablet form. These pills can be in different colors with images on them. Molly is taken in a gel capsule or snorted. What does MDMA do to the body and mind? • As a stimulant drug, it increases heart rate and blood pressure. Users may experience muscle tension, involuntary teeth clenching, nausea, blurred vision, faintness, chills, or sweating • It produces feelings of increased energy, euphoria, emotional warmth, empathy, and distortions in sensory and time perception. • Feelings of sadness, anxiety, depression and memory difficulties are other effects. • It can seriously deplete serotonin levels in the brain, causing confusion and sleep problems. Did you know? • DEA has labeled MDMA as a Schedule I drug, meaning its abuse potential is high and it has no approved medical use. It is illegal in the U.S. • In high doses, MDMA can affect the body’s ability to regulate temperature, which can lead to serious health complications and possible death. • Teens are using less MDMA. Teens decreased their past year use of MDMA from 1.9% in 2010 to 1.2% of teens using 2012. -

Psychoactive Substances and Transpersonal States
TRANSPERSONAL PSYCHOLOGY RESEARCH REVIEW: PSYCHOACTIVE SUBSTANCES AND TRANSPERSONAL STATES David Lukoff San Francisco, California Robert Zanger Los Angeles, California Francis Lu San Francisco, California This "Research Review" covers recent trends in researching psychoactive substances and trans personal states of conscious ness during the past ten years. In keeping with the stated goals of this section of the Journal to promote research in transpersonal psychology, the focus is on the methods and trends designs which are being employed in investigations rather than during the findings on this topic. However, some recently published the books and monographs provide good summaries of the recent last findings relevant to understanding psychoactive substances ten (Cohen & Krippner, 1985b; Dobkin de Rios, 1984;Dobkin de years Rios & Winkelman, 1989b; Ratsch, 1990; Reidlinger, 1990). Because researching psychoactive substances is most broadly a cross-disciplinary venture, only a small portion of the research reviewed below was conducted by persons who consider The authors wish to acknowledge the assistance of Bruce Flath, head librarian at the California Institute of Integral Studies, San Francisco in conducting the computer bibliographic searches used in preparation of this article. The authors also wish to thank Marlene Dobkin de Rios, Stanley Krippner, Christel Lukoff, Dennis McKenna, Terence McKenna, Ralph Metzner, Donald Rothberg and Ilene Serlin for their valuable comments on earlier drafts of this article. Copyright © 1990 Transpersonal Institute The Journal of Transpersonal Psychology. 1990, Vol. 22, No.2 107 themselves transpersonal psychologists. As Vaughan (1984) has noted, "The transpersonal perspective is a meta-perspec tive, an attempt to learn from all different disciplines . emerging from the needed integration of ancient wisdom and modern science. -

CONTROLLED SUBSTANCE, DRUG, DEVICE and COSMETIC ACT - SCHEDULE I CONTROLLED SUBSTANCES Act of Jun
CONTROLLED SUBSTANCE, DRUG, DEVICE AND COSMETIC ACT - SCHEDULE I CONTROLLED SUBSTANCES Act of Jun. 23, 2011, P.L. 36, No. 7 Cl. 35 Session of 2011 No. 2011-7 SB 1006 AN ACT Amending the act of April 14, 1972 (P.L.233, No.64), entitled "An act relating to the manufacture, sale and possession of controlled substances, other drugs, devices and cosmetics; conferring powers on the courts and the secretary and Department of Health, and a newly created Pennsylvania Drug, Device and Cosmetic Board; establishing schedules of controlled substances; providing penalties; requiring registration of persons engaged in the drug trade and for the revocation or suspension of certain licenses and registrations; and repealing an act," further providing for Schedule I controlled substances. The General Assembly of the Commonwealth of Pennsylvania hereby enacts as follows: Section 1. Section 4(1) of the act of April 14, 1972 (P.L.233, No.64), known as The Controlled Substance, Drug, Device and Cosmetic Act, amended November 24, 1999 (P.L.894, No.55), is amended to read: Section 4. Schedules of Controlled Substances.--The following schedules include the controlled substances listed or to be listed by whatever official name, common or usual name, chemical name, or trade name designated. (1) Schedule I--In determining that a substance comes within this schedule, the secretary shall find: a high potential for abuse, no currently accepted medical use in the United States, and a lack of accepted safety for use under medical supervision. The following controlled substances are included in this schedule: (i) Any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, whenever the existence of such isomers, esters, ethers and salts is possible within the specific chemical designation: 1. -

The Canadian Cannabis Story
A Generational Investment Opportunity THE CANADIAN CANNABIS STORY JOIN THE CONVERSATION / Echelon Wealth Partners echelonpartners.com TABLE OF CONTENTS 3 The Canadian Cannabis Story: A Generational Investment Opportunity 4 Cannabis: A Brief History 5 The Many Forms of Cannabis 5 An Increase in Legal Cannabis-based Products 5 Medical Use 8 Cannabis as an Opiod Alternative 11 Cannabis and Canada: A Strong Growth Story 14 A Global Cannabis Boom: The Next Stage 17 Canadian Cannabis Stocks - An Investment Opportunity to Consider 19 Endnotes echelonpartners.com 2 The Canadian Cannabis Story: A Generational Investment Opportunity THE CANADIAN CANNABIS STORY: A GENERATIONAL INVESTMENT OPPORTUNITY By Echelon Wealth Partners The Canadian Cannabis Story aims to provide readers with a comprehensive look at the cannabis market in Canada through its history, growth, and various production sectors to illuminate the investment opportunity this sector will afford in a rapidly growing global market. The increasing trend in cannabis decriminalization and legalization, both in North America and around the world, has awakened the interest of the investment community. The unique and potential medicinal properties of cannabis and its versatility for other commercial uses are considered the harbingers of an investment with significant growth potential. Canada legalized marijuana for recreational use on October 17, 2018 echelonpartners.com 3 The Canadian Cannabis Story: A Generational Investment Opportunity CANNABIS A Brief History Marijuana is produced from the flower and leaves of cannabis plants, which grow naturally in humid temperate conditions on all continents.1 The two most important varieties of the cannabis plant are sativa and indica, with hemp being a specific species of the sativa plant. -

The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 6-2020 The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting Robert J. Kohler Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Biological Psychology Commons Recommended Citation Kohler, Robert J., "The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting" (2020). Dissertations. 3565. https://scholarworks.wmich.edu/dissertations/3565 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING by Robert J. Kohler A dissertation submitted to the Graduate College In partial fulfillment of the requirements for the degree of Doctor of Philosophy Psychology Western Michigan University June 2020 Doctoral Committee: Lisa Baker, Ph.D., Chair Anthony DeFulio, Ph.D. Cynthia Pietras, Ph.D. John Spitsbergen, Ph.D. Copyright by Robert J. Kohler 2020 THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING Robert J. Kohler, Ph.D. Western Michigan University, 2020 The resurgence of Lysergic Acid Diethylamide (LSD) as a therapeutic tool requires a revival in research, both basic and clinical, to bridge gaps in knowledge left from a previous generation of work. Currently, no study has been published with the intent of establishing optimal microdose concentrations of LSD in an animal model. -
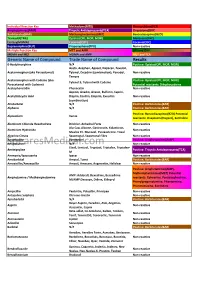
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied.