Stable Derivatives of Thromboxane A2 with Differential Effects on Platelets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Thrombin and Thromboxane A2 in Reocclusion Following Coronary
Proc. Natl. Acad. Sci. USA Vol. 86, pp. 7585-7589, October 1989 Medical Sciences Role of thrombin and thromboxane A2 in reocclusion following coronary thrombolysis with tissue-type plasminogen activator (thrombolytic therapy/coronary thrombosis/platelet activation/reperfusion) DESMOND J. FITZGERALD*I* AND GARRET A. FITZGERALD* Divisions of *Clinical Pharmacology and tCardiology, Vanderbilt University, Nashville, TN 37232 Communicated by Philip Needleman, June 28, 1989 (receivedfor review April 12, 1989) ABSTRACT Reocclusion of the coronary artery occurs against the prothrombinase formed on the platelet surface after thrombolytic therapy of acute myocardlal infarction (13) and the neutralization ofheparin by platelet factor 4 (14) despite routine use of the anticoagulant heparin. However, and thrombospondin (15), proteins released by activated heparin is inhibited by platelet activation, which is greatly platelets. enhanced in this setting. Consequently, it is unclear whether To address the role of thrombin during coronary throm- thrombin induces acute reocclusion. To address this possibility, bolysis, we have examined the effect of a specific thrombin we examined the effect of argatroban {MCI9038, (2R,4R)- inhibitor, argatroban {MCI9038, (2R,4R)-4-methyl-1-[N-(3- 4-methyl-l-[Na-(3-methyl-1,2,3,4-tetrahydro-8-quinolinesulfo- methyl-1,2,3,4-tetrahydro-8-quinolinesulfonyl)-L-arginyl]-2- nyl)-L-arginyl]-2-piperidinecarboxylic acid}, a specific throm- piperidinecarboxylic acid} on the response to tissue plasmin- bin inhibitor, on the response to tissue-type plasminogen ogen activator (t-PA) in a closed-chest canine model of activator in a dosed-chest canine model of coronary thrombo- coronary thrombosis. MCI9038, an argimine derivative which sis. MCI9038 prolonged the thrombin time and shortened the binds to a hydrophobic pocket close to the active site of time to reperfusion (28 + 2 min vs. -

AA Metabolism, 139 A2b1, 69, 70, 318–323 ABCB1 Gene, 176 Abciximab
Index A aIIbb3, 60, 62, 69, 70, 73, 74, 79, 80 AA. See Arachidonic acid (AA) AJW202, 293 AA metabolism, 139 Akt-1, 345 a2b1, 69, 70, 318–323 Akt-2, 345 ABCB1 gene, 176 ALX-0081, 296 Abciximab, 206–208, 497, 500, 501, 504–505, ALX-0681, 298 509, 513, 514, 525 Anagrelide, 227, 229 approval, 206 Ankle brachial indexes (ABIs), 549, 587, EPIC trial, 207 595–597 EPILOG study, 207 framingham risk score, 551 EPISTENT study, 207 screening, 550 platelet binding, 207 sensitivity, 551 thrombocytopenia, 206 specificity, 551 in unstable angina, 208 vascular risk, 550 ABIs. See Ankle brachial indexes (ABIs) Antagomirs, 439 Acetylsalicylic acid (aspirin), 137–158 Antibodies, 317 ACS. See Acute coronary syndromes (ACS) Anticoagulants, 525, 537 Acute coronary syndromes (ACS), 288 Anticoagulation, 533, 534 Acute ischemic stroke, 143, 149–150 Antioxidant, 233 Acute myocardial infarction, 142, 143, 149, Antiplatelet agents, 263, 553 153, 157 Antithrombotic Trialist’s ADAMTS-13, 93 Collaboration, 553 Adenosine diphosphate (ADP), 37, 88, 96, aspirin, 553 166, 473 clopidogrel, 553 Adenosine triphosphate (ATP), 37 dipyridamole, 553 Adhesion, 90, 112–118, 125 meta-analysis, 560 Adhesion molecules infiltration, 264 picotamide, 553 Adiponectin, 315 risk reduction, 553 ADP. See Adenosine diphosphate (ADP) ticlopidine, 553 ADP inhibitors (or P2Y12 blockers), 497–499, vascular events, 553 501–505, 507–509, 513, 514 Antiplatelet therapy, 472 ADP-receptor antagonists, 169 Apixaban, 535, 539 Adverse effects, 153–156 Aptamers, 299–301 Aegyptin, 327 Arachidonic acid (AA), 474 P. Gresele et al. (eds.), Antiplatelet Agents, Handbook of Experimental 607 Pharmacology 210, DOI 10.1007/978-3-642-29423-5, # Springer-Verlag Berlin Heidelberg 2012 608 Index ARC1779, 299–300 TXS signaling, 279 ARC15105, 300 Cardiovascular death, 248 AR-C69931MX, 456 Carotid endarterectomy (ACE) inhibition, Aspirin, 474, 496, 498–499, 501–502, 143, 156 504–507, 511–514, 520, 522, Carotid stenosis, 523 527, 531, 533–537, 539, CCBs. -

Eicosanoids & Platelet Activating Factor
Eicosanoids & Platelet Activating Factor Prof. Sanjay Khattri Dept. of Pharmacology & Therapeutics King George’s Medical University, Lucknow 1 Autacoid These are the substances produced by wide variety of cells that act locally at the site of production. (local hormones) 2 Mediators of Inflammation and Immune reaction 1. Vasoactive amines (Histamine and Serotonin) 2.Eicosanoids 3.Platlet Activating Factor 4.Bradykinins 4.Nitric Oxide 5.Neuropeptides 6.Cytokinines 3 EICOSANOIDS PGs, TXs and LTs are all derived from eicosa (referring to 20 C atoms) tri/tetra/ penta enoic acids. Therefore, they can be collectively called eicosanoids. Major source: 5,8,11,14 eicosa tetraenoic acid (arachidonic acid). Other eicosanoids of increasing interest are: lipoxins and resolvins. The term prostanoid encompasses both prostaglandins and thromboxanes. 4 EICOSANOIDS Contd…. In most instances, the initial and rate-limiting step in eicosanoid synthesis is the liberation of intracellular arachidonate, usually in a one-step process catalyzed by the enzyme phospholipase A2 (PLA2). PLA2 generates not only arachidonic acid but also lysoglyceryl - phosphorylcholine (lyso-PAF), the precursor of platelet activating factor (PAF). 5 EICOSANOIDS Contd…. Corticosteroids inhibit the enzyme PLA2 by inducing the production of lipocortins (annexins). The free arachidonic acid is metabolised separately (or sometimes jointly) by several pathways, including the following: Cyclo-oxygenase (COX)- Two main isoforms exist, COX-1 and COX-2 Lipoxygenases- Several subtypes, which -

Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome
International Journal of Molecular Sciences Review Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome Stefano Turolo 1,* , Alberto Edefonti 1 , Alessandra Mazzocchi 2, Marie Louise Syren 2, William Morello 1, Carlo Agostoni 2,3 and Giovanni Montini 1,2 1 Fondazione IRCCS Ca’ Granda-Ospedale Maggiore Policlinico, Pediatric Nephrology, Dialysis and Transplant Unit, Via della Commenda 9, 20122 Milan, Italy; [email protected] (A.E.); [email protected] (W.M.); [email protected] (G.M.) 2 Department of Clinical Sciences and Community Health, University of Milan, 20122 Milan, Italy; [email protected] (A.M.); [email protected] (M.L.S.); [email protected] (C.A.) 3 Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Pediatric Intermediate Care Unit, 20122 Milan, Italy * Correspondence: [email protected] Abstract: Studies concerning the role of arachidonic acid (AA) and its metabolites in kidney disease are scarce, and this applies in particular to idiopathic nephrotic syndrome (INS). INS is one of the most frequent glomerular diseases in childhood; it is characterized by T-lymphocyte dysfunction, alterations of pro- and anti-coagulant factor levels, and increased platelet count and aggregation, leading to thrombophilia. AA and its metabolites are involved in several biological processes. Herein, Citation: Turolo, S.; Edefonti, A.; we describe the main fields where they may play a significant role, particularly as it pertains to their Mazzocchi, A.; Syren, M.L.; effects on the kidney and the mechanisms underlying INS. AA and its metabolites influence cell Morello, W.; Agostoni, C.; Montini, G. -
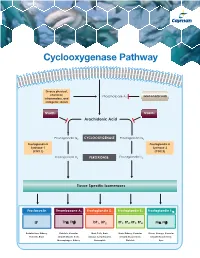
Cyclooxygenase Pathway
Cyclooxygenase Pathway Diverse physical, chemical, Phospholipase A Glucocorticoids inflammatory, and 2 mitogenic stimuli NSAIDs NSAIDs Arachidonic Acid Prostaglandin G2 CYCLOOXYGENASE Prostaglandin G2 Prostaglandin H Prostaglandin H Synthase-1 Synthase-2 (COX 1) (COX 2) Prostaglandin H2 PEROXIDASE Prostaglandin H2 Tissue Specific Isomerases Prostacyclin Thromboxane A2 Prostaglandin D2 Prostaglandin E2 Prostaglandin F2α IP TPα, TPβ DP1, DP2 EP1, EP2, EP3, EP4 FPα, FPβ Endothelium, Kidney, Platelets, Vascular Mast Cells, Brain, Brain, Kidney, Vascular Uterus, Airways, Vascular Platelets, Brain Smooth Muscle Cells, Airways, Lymphocytes, Smooth Muscle Cells, Smooth Muscle Cells, Macrophages, Kidney Eosinophils Platelets Eyes Prostacyclin Item No. Product Features Prostacyclin (Prostaglandin I2; PGI2) is formed from arachidonic acid primarily in the vascular endothelium and renal cortex by sequential 515211 6-keto • Sample Types: Culture Medium | Plasma Prostaglandin • Measure 6-keto PGF levels down to 6 pg/ml activities of COX and prostacyclin synthase. PGI2 is non-enzymatically 1α F ELISA Kit • Incubation : 18 hours | Development: 90-120 minutes | hydrated to 6-keto PGF1α (t½ = 2-3 minutes), and then quickly converted 1α Read: Colorimetric at 405-420 nm to the major metabolite, 2,3-dinor-6-keto PGF1α (t½= 30 minutes). Prostacyclin was once thought to be a circulating hormone that regulated • Assay 24 samples in triplicate or 36 samples in duplicate platelet-vasculature interactions, but the rate of secretion into circulation • NOTE: A portion of urinary 6-keto PGF1α is of renal origin coupled with the short half-life indicate that prostacyclin functions • NOTE : It has been found that normal plasma levels of 6-keto PGF may be low locally. -

Vasoactive Responses of U46619, PGF2 , Latanoprost, and Travoprost
Vasoactive Responses of U46619, PGF2␣, Latanoprost, and Travoprost in Isolated Porcine Ciliary Arteries Ineta Vysniauskiene, Reto Allemann, Josef Flammer, and Ivan O. Haefliger PURPOSE. To compare the vasoactive properties of the prosta- these responses can be modulated by SQ 29548 (TP-receptor noids U46619 (thromboxane A2 analogue), prostaglandin F2␣ antagonist) or AL-8810 (FP-receptor antagonist). (PGF2␣), latanoprost free acid, and travoprost free acid in isolated porcine ciliary arteries. METHODS. In a myograph system (isometric force measure- MATERIAL AND METHODS ment), quiescent vessels were exposed (cumulatively) to U46619, PGF , latanoprost, or travoprost (0.1 nM–0.1 mM). 2␣ Vessels Preparation Experiments were also conducted in the presence of SQ 29548 (TP-receptor antagonist; 3–10 M) or AL-8810 (FP-receptor Porcine eyes were obtained from an abattoir immediately after death. antagonist; 3–30 M). Contractions were expressed as the In cold modified Krebs-Ringer solution (NaCl 118 mM, KCl 4.7 mM, percentage of 100 mM potassium chloride-induced contrac- CaCl2 2.5 mM, K2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25 mM, tions. glucose 11.1 mM, and EDTA 0.026 mM), ciliary arteries were dissected 5 RESULTS. In quiescent vessels, contractions (concentration–re- and cut into 2-mm segments. In an organ chamber, two 45- m Ϯ tungsten wires were passed through the vessel’s lumen and attached to sponse curves) induced by (0.1 mM) PGF2␣ (87.9% 3.5%), U46619 (66.7% Ϯ 4.1%), and latanoprost (62.9% Ϯ 3.6%) were a force transducer for isometric force measurements (Myo-Interface; JP more pronounced (P Յ 0.001) than those induced by tra- Trading, Aarhus, Denmark). -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Activation of the Murine EP3 Receptor for PGE2 Inhibits Camp Production and Promotes Platelet Aggregation
Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre, … , Thomas M. Coffman, Beverly H. Koller J Clin Invest. 2001;107(5):603-610. https://doi.org/10.1172/JCI10881. Article The importance of arachidonic acid metabolites (termed eicosanoids), particularly those derived from the COX-1 and COX-2 pathways (termed prostanoids), in platelet homeostasis has long been recognized. Thromboxane is a potent agonist, whereas prostacyclin is an inhibitor of platelet aggregation. In contrast, the effect of prostaglandin E2 (PGE2) on platelet aggregation varies significantly depending on its concentration. Low concentrations of PGE2 enhance platelet aggregation, whereas high PGE2 levels inhibit aggregation. The mechanism for this dual action of PGE2 is not clear. This study shows that among the four PGE2 receptors (EP1–EP4), activation of EP3 is sufficient to mediate the proaggregatory actions of low PGE2 concentration. In contrast, the prostacyclin receptor (IP) mediates the inhibitory effect of higher PGE2 concentrations. Furthermore, the relative activation of these two receptors, EP3 and IP, regulates the intracellular level of cAMP and in this way conditions the response of the platelet to aggregating agents. Consistent with these findings, loss of the EP3 receptor in a model of venous inflammation protects against formation of intravascular clots. Our results suggest that local production of PGE2 during an inflammatory process can modulate ensuing platelet responses. Find the latest version: https://jci.me/10881/pdf Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation Jean-Etienne Fabre,1 MyTrang Nguyen,1 Krairek Athirakul,2 Kenneth Coggins,1 John D. -

Antiplatelet Effects of Prostacyclin Analogues: Which One to Choose in Case of Thrombosis Or Bleeding? Sylwester P
INTERVENTIONAL CARDIOLOGY Cardiology Journal 20XX, Vol. XX, No. X, XXX–XXX DOI: 10.5603/CJ.a2020.0164 Copyright © 20XX Via Medica REVIEW ARTICLE ISSN 1897–5593 eISSN 1898–018X Antiplatelet effects of prostacyclin analogues: Which one to choose in case of thrombosis or bleeding? Sylwester P. Rogula1*, Hubert M. Mutwil1*, Aleksandra Gąsecka1, Marcin Kurzyna2, Krzysztof J. Filipiak1 11st Chair and Department of Cardiology, Medical University of Warsaw, Poland 2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Center of Postgraduate Education Medical, European Health Center Otwock, Poland Abstract Prostacyclin and analogues are successfully used in the treatment of pulmonary arterial hypertension (PAH) due to their vasodilatory effect on pulmonary arteries. Besides vasodilatory effect, prostacyclin analogues inhibit platelets, but their antiplatelet effect is not thoroughly established. The antiplatelet effect of prostacyclin analogues may be beneficial in case of increased risk of thromboembolic events, or undesirable in case of increased risk of bleeding. Since prostacyclin and analogues differ regarding their potency and form of administration, they might also inhibit platelets to a different extent. This review summarizes the recent evidence on the antiplatelet effects of prostacyclin and analogue in the treatment of PAH, this is important to consider when choosing the optimal treatment regimen in tailoring to an individual patients’ needs. (Cardiol J 20XX; XX, X: xx–xx) Key words: prostacyclin analogues, pulmonary arterial hypertension, platelets, antiplatelet effect, thrombosis, bleeding Introduction tiproliferative effects [4]. The main indication for PGI2 and analogues is advanced pulmonary arterial Since 1935 when prostaglandin was isolated hypertension (PAH) and peripheral vascular disor- for the first time [1], many scientists have focused ders [5]. -

2374 Supplementary Drugs and Other Substances
2374 Supplementary Drugs and Other Substances 5. Burdock GA. Review of the biological properties and toxicity of Pharmacokinetics reduced to the endoperoxide prostaglandin H2 (PGH2). Prostag- bee propolis (propolis). Food Chem Toxicol 1998; 36: 347–63. Propylene glycol is rapidly absorbed from the gastrointestinal landin H2 is then converted to the primary prostaglandins pros- 6. Lieberman HD, et al. Allergic contact dermatitis to propolis in a tract. There is evidence of topical absorption when applied to taglandin D2, prostaglandin E2, and prostaglandin F2 , to throm- violin maker. J Am Acad Dermatol 2002; 46 (suppl): S30–S31. damaged skin. boxane A2 (TXA2) via the enzyme thromboxane synthetase, or 7. Giusti F, et al. Sensitization to propolis in 1255 children under- It is extensively metabolised in the liver primarily by oxidation to prostacyclin (PGI2) via the enzyme prostacyclin synthetase. going patch testing. Contact Dermatitis 2004; 51: 255–8. to lactic and pyruvic acid and is also excreted in the urine These products are further metabolised and rapidly inactivated in 8. Walgrave SE, et al. Allergic contact dermatitis from propolis. unchanged. the body. Dermatitis 2005; 16: 209–15. The secondary prostaglandins, prostaglandin A (PGA ), pros- 9. Majiene D, et al. Antifungal and antibacterial activity of propo- ◊ References. 2 2 lis. Curr Nutr Food Sci 2007; 3: 304–8. 1. Yu DK, et al. Pharmacokinetics of propylene glycol in humans taglandin B2 (PGB2), and prostaglandin C2 (PGC2) are derived during multiple dosing regimens. J Pharm Sci 1985; 74: 876–9. from prostaglandin E2, but are formed during extraction and Preparations 2. Speth PAJ, et al. -

COX-1 and COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr
COX-1 And COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr. David Nelson (Professor of Biochemistry, University of Wisconsin- (Student, University of Wisconsin-Madison) Center for Inhibited by NSAIDS (Nonsteroidal Anti-inflammatory Drugs) BioMolecular Madison West High School: Audra Amasino, Yuting Deng, Samuel Huang, Iris Lee, Adeyinka Lesi, Yaoli Pu, and Peter Vander Velden Modeling Advisor: Gary Graper, Teacher Emeritus, University of Wisconsin-Madison Mentors: Dr. David Nelson, Professor of Biochemistry, and Basudeb Bhattacharyya, Student, University of Wisconsin-Madison Abstract Prostaglandin Hormone Synthases (COX-1 and COX-2) are enzymes that produce prostaglandins. Prostaglandins are (1) Structure (3) Cyclooxygenase Active Sites responsible for fever, pain, and inflammation, but also the (5) Drugs maintenance of the lining of the stomach and prevention of In the pictures below, the heme is orange, the ulceration. COX-1 is found mainly in the gastrointestinal lining, hydrophobic knob is yellow, and the amino acids in the The three drugs below are all nonsteroidal anti- and COX-2 at sites of inflammation. NSAIDS (Nonsteroidal anti- Cyclooxygenase active site are colored in CPK (red for inflammatory drugs (NSAIDS). The first two NSAIDS, inflammatory drugs) such as aspirin, ibuprofen, naproxen, and oxygen, blue for nitrogen, and gray for carbon). aspirin and ibuprofen, are called nonselective Cox flurbiprofen inhibit both COX-1 and COX-2, and are taken inhibitors since they affect both COX-1 and COX-2 regularly by over 33 million Americans for pain and substantially (note their high COX-2/COX-1 effect inflammation. Some 10%-50% of these users suffer ratios). -

Misoprostol Induces Relaxation of Human Corpus Cavernosum Smooth Muscle: Comparison to Prostaglandin E1
International Journal of Impotence Research (2000) 12, 107±110 ß 2000 Macmillan Publishers Ltd All rights reserved 0955-9930/00 $15.00 www.nature.com/ijir Misoprostol induces relaxation of human corpus cavernosum smooth muscle: comparison to prostaglandin E1 RB Moreland1, NN Kim1, A Nehra2, BG Parulkar3 and A Traish1,4* 1Department of Urology, Boston University School of Medicine, Boston, MA 02118, USA; 2Department of Urology, Mayo Clinic and Foundation, Rochester, MN 55905, USA; 3Department of Urology, University of Massachusetts Medical Center, Worcester, MA 01604, USA; and 4Department of Biochemistry, Boston University School of Medicine, Boston, MA 02118, USA Prostaglandin E1 (PGE1) relaxes trabecular smooth muscle by interacting with speci®c G-protein coupled receptors on human corpus cavernosum smooth muscle and increasing intracellular synthesis of cAMP. Misoprostol (CytotecTM), is an oral prostaglandin E analogue. The purpose of this study was to compare the functional activity of misoprostol with PGE1 in human corpus cavernosum and cultured human corpus cavernosum smooth muscle cells. Misoprostol, misoprostol free acid or PGE1 induced dose-dependent relaxations in strips of human corpus cavernosum. At concentrations greater than 1076 M, tissue recontraction was observed with all three agents. This was abrogated by pretreatment with the thromboxane A2 receptor antagonist SQ29,548. From these observations, we conclude that misoprostol is activated by human corpus cavernosum in situ and relaxes phenylephrine-precontrated tissue