Fatty Alcohols – a Review of Their Natural Synthesis and Environmental Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalyst in Basic Oleochemicals
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Diponegoro University Institutional Repository Bulletin of Chemical Reaction Engineering & Catalysis, 2(2-3), 2007, 22-31 Catalyst in Basic Oleochemicals Eva Suyenty, Herlina Sentosa, Mariani Agustine, Sandy Anwar, Abun Lie, and Erwin Sutanto * Research and Development Department, PT. Ecogreen Oleochemicals Jln. Pelabuhan Kav 1, Kabil, Batam 29435, Telp/Fax: (0778)711374 Presented at Symposium and Congress of MKICS 2007, 18-19 April 2007, Semarang, Indonesia Abstract Currently Indonesia is the world largest palm oil producer with production volume reaching 16 million tones per annum. The high crude oil and ethylene prices in the last 3 – 4 years contribute to the healthy demand growth for basic oleochemicals: fatty acids and fatty alcohols. Oleochemicals are starting to replace crude oil derived products in various applications. As widely practiced in petrochemical industry, catalyst plays a very important role in the production of basic oleochemicals. Catalytic reactions are abound in the production of oleochemicals: Nickel based catalysts are used in the hydrogenation of unsaturated fatty ac- ids; sodium methylate catalyst in the transesterification of triglycerides; sulfonic based polystyrene resin catalyst in esterification of fatty acids; and copper chromite/copper zinc catalyst in the high pressure hydro- genation of methyl esters or fatty acids to produce fatty alcohols. To maintain long catalyst life, it is crucial to ensure the absence of catalyst poisons and inhibitors in the feed. The preparation methods of nickel and copper chromite catalysts are as follows: precipitation, filtration, drying, and calcinations. Sodium methy- late is derived from direct reaction of sodium metal and methanol under inert gas. -

Study of Fatty Acid and Fatty Alcohol Formation from Hydrolysis of Rice Bran Wax Kelly L
1747 A publication of CHEMICAL ENGINEERING TRANSACTIONS The Italian Association VOL. 32, 2013 of Chemical Engineering Online at: www.aidic.it/cet Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright © 2013, AIDIC Servizi S.r.l., ISBN 978-88-95608-23-5; ISSN 1974-9791 Study of Fatty Acid and Fatty Alcohol Formation from Hydrolysis of Rice Bran Wax Kelly L. Tronia, Simone M. Silvab, Antonio J.A. Meirellesb, Roberta Ceriani*a a Faculty of Chemical Engineering, University of Campinas (UNICAMP), Zip Code, 13083-852, Campinas, São Paulo, Brazil b Faculty of Food Engineering, University of Campinas (UNICAMP), Zip Code, 13083-862, Campinas, São Paulo, Brazil [email protected] Rice bran wax is waste material of dewaxing process in oil refining. Dewaxing is accomplished by cooling and filtrating for separating wax from the oil to avoid turbidity in the final product. The dewaxing residue may have 20 up to 80 wt% of oil, followed by a main fraction of waxes, free fatty alcohols, free fatty acids and hydrocarbons. The wax fraction of the residue is composed by long-chain fatty alcohols esterified with long-chain fatty acids. Considering that rice bran oil has 4 – 6 wt% of wax, a large amount of this natural source of fatty compounds is undervalued. Noweck and Rider (1987) describe a process based on hydrolysis of waxes with sodium hydroxide, followed by distillation of fatty alcohols and soap. According to best of our knowledge no work has been reported on the formation of fatty acids and alcohols from the hydrolysis of the dewaxing residue using supersaturated stripping steam under high temperatures and high vacuum. -

1.1 10 Oxidation of Alcohols and Aldehydes
1.1 10 Oxidation of alcohols and aldehydes By the end of this spread, you should be able to … 1Describe the oxidation of primary alcohols to form aldehydes and carboxylic acids. 1Describe the oxidation of secondary alcohols to form ketones. 1Describe the oxidation of aldehydes to form carboxylic acids. Key definition Oxidation of alcohols You will recall from your AS chemistry studies that primary and secondary alcohols can A redox reaction is one in which both reduction and oxidation take place. be oxidised using an oxidising agent. s !SUITABLEOXIDISINGAGENTISASOLUTIONCONTAININGACIDIlEDDICHROMATEIONS + 2− H /Cr2O7 . s 4HEOXIDISINGMIXTURECANBEMADEFROMPOTASSIUMDICHROMATE +2Cr2O7, and sulfuric acid, H2SO. During the reaction, the acidified potassium dichromate changes from orange to green. Remember that tertiary alcohols are not oxidised by acidified dichromate ions. Primary alcohols Primary alcohols can be oxidised to aldehydes and to carboxylic acids (see AS Chemistry spread 2.2.3). H H H O H O Oxidation Oxidation H CCOH H CC H CC H H H H H OH Primary alcohol Aldehyde Carboxylic acid Figure 1 Ethanol oxidised to ethanal, and finally to ethanoic acid The equation for the oxidation of ethanol to the aldehyde ethanal is shown below. Note that the oxidising agent is shown as [O] – this simplifies the equation. CH3CH2OH + [O] }m CH3CHO + H2O When preparing aldehydes in the laboratory, you will need to distil the aldehyde from the reaction mixture as it is formed. This prevents the aldehyde from being oxidised further to a carboxylic acid. Key definition When making the carboxylic acid, the reaction mixture is usually heated under reflux before distilling the product off. -

( 12 ) United States Patent ( 10 ) Patent No .: US 10,751,310 B2 Freeman Et Al
US010751310B2 ( 12 ) United States Patent ( 10 ) Patent No .: US 10,751,310 B2 Freeman et al . ( 45 ) Date of Patent : Aug. 25 , 2020 ( 54 ) PREVENTION , TREATMENT AND ( 56 ) References Cited REVERSAL OF DISEASE USING THERAPEUTICALLY EFFECTIVE U.S. PATENT DOCUMENTS AMOUNTS OF DICARBOXYLIC ACID 3,527,789 A 9/1970 Payne COMPOUNDS 4,166,913 A 9/1979 Kesling , Jr. et al . 6,528,499 B1 * 3/2003 Kozikowski C07C 59/347 ( 71 ) Applicant: UNIVERSITY OF 514/574 8,324,277 B2 12/2012 Freeman PITTSBURGH — OF THE 8,735,449 B2 5/2014 Freeman COMMONWEALTH SYSTEM OF 9,066,902 B2 6/2015 Freeman et al . HIGHER EDUCATION , Pittsburgh , 9,186,408 B2 11/2015 Freeman et al . PA (US ) 9,700,534 B2 7/2017 Freeman et al . 9,750,725 B2 9/2017 Freeman et al . 10,213,417 B2 2/2019 Freeman et al . ( 72 ) Inventors : Bruce A. Freeman , Pittsburgh , PA 10,258,589 B2 4/2019 Freeman et al . ( US ) ; Francisco J. Schopfer , 2015/0018417 Al 1/2015 Freeman et al . Pittsburgh , PA ( US ) FOREIGN PATENT DOCUMENTS ( 73 ) Assignee : University of Pittsburgh — of the CN 103705499 4/2014 Commonwealth System of Higher DE 102011118462 5/2013 Education , Pittsburgh , PA ( US ) GB 1153464 5/1969 WO WO 2002/022627 3/2002 WO WO 2009/017802 2/2009 ( * ) Notice : Subject to any disclaimer , the term of this WO WO 2009/112455 9/2009 patent is extended or adjusted under 35 WO WO 2010/005521 1/2010 U.S.C. 154 ( b ) by 0 days . WO WO 2010/014889 2/2010 WO WO 2011/014261 2/2011 WO WO 2013/116753 8/2013 ( 21 ) Appl. -
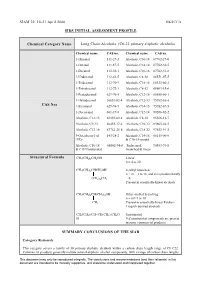
Long Chain Alcohols (C6-22 Primary Aliphatic Alcohols)
SIAM 22, 18-21 April 2006 UK/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Long Chain Alcohols (C6-22 primary aliphatic alcohols) Chemical name CAS no. Chemical name CAS no. 1-Hexanol 111-27-3 Alcohols, C16-18 67762-27-0 1-Octanol 111-87-5 Alcohols, C14-18 67762-30-5 1-Decanol 112-30-1 Alcohols, C10-16 67762-41-8 1-Undecanol 112-42-5 Alcohols, C8-18 68551-07-5 1-Tridecanol 112-70-9 Alcohols, C14-16 68333-80-2 1-Tetradecanol 112-72-1 Alcohols, C6-12 68603-15-6 1-Pentadecanol 629-76-5 Alcohols, C12-16 68855-56-1 1-Hexadecanol 36653-82-4 Alcohols, C12-13 75782-86-4 CAS Nos 1-Eicosanol 629-96-9 Alcohols, C14-15 75782-87-5 1-Docosanol 661-19-8 Alcohols, C12-14 80206-82-2 Alcohols, C12-15 63393-82-8 Alcohols, C8-10 85566-12-7 Alcohols, C9-11 66455-17-2 Alcohols, C10-12 85665-26-5 Alcohols, C12-18 67762-25-8 Alcohols, C18-22 97552-91-5 9-Octadecen-1-ol 143-28-2 Alcohols, C14-18. 68155-00-0 (9Z)- & C16-18-unsatd Alcohols, C16-18 68002-94-8 Tridecanol, 90583-91-8 & C18 Unsaturated branched & linear Structural Formula CH3(CH2)nCH2OH Linear n = 4 to 20 CH3(CH2)nCHCH2OH 2-Alkyl branched n + m = 3 to 18, and m is predominantly (CH2)mCH3 = 0. Present in essentially-linear alcohols CH3(CH2)nCH(CH2)mOH Other-methyl branching n + m= 9 or 10 CH3 Present in essentially-linear Fischer- Tropsch derived alcohols CH3(CH2)7CH=CH(CH2)7CH2O Unsaturated H 9-Z unsaturated components are present in some commercial products. -

U Nited States Patent (19)
United States Patent (19) 11) Patent Number: 5,059,718 Vargas, Jose M. et al. 45) Date of Patent: Oct. 22, 1991 54) OXO PROCESS FOR INCREASING YIELD 4,656,215 4/1987 Haninet al. ......................... 524/376 OF OXO ALCOHOL 4,658,068 4/1987 Hanin ..... 568/451 4,683,343 7/1987 Hanin et al. ......................... 568/594 (75) Inventors: Vargas, Jose M., Baton Rouge, La., Jean A. Hanin, Rixensart, Belgium; FOREIGN PATENT DOCUMENTS John C. Reisch, Hilton Head, S.C. 756877 3/1971 Belgium .............................. 568/881 (73) Assignee: Exxon Chemical Patents Inc., OTHER PUBLICATIONS Linden, N.J. Kirk-Othmer, Encyclopedia of Chemical Technology, 21 Appl. No.: 586,906 vol. 16, 3rd Edition, John Wiley & Sons, pp. 637-653, 22) Filed: Sep. 24, 1990 1981. (51) Int. Cl. ....................... C07C 29/14: CO7C 29/74 Primary Examiner-Werren B. Lone 52) U.S. C. .................................... 568/881; 568/810; Attorney, Agent, or Firm-J. J. Mahon 568/880 (58) Field of Search ................ 568/462, 880, 881, 810 (57) ABSTRACT References Cited Improved oxo process for preparing higher alcohols by (56) hydrolyzing prior to hydrogenation a demetalled hy U.S. PATENT DOCUMENTS droformylation reaction product in the presence of an 3,925,490 12/1975 Reich et al. ......................... 568/881 alumina catalyst having a surface area between 40 to 3,935,285 1/1976 Tunnes et al. ... 260/638 about 60 m2/g. 4,401,834 8/1983 King .................... ... 568/881 4,404,119 9/1983 Lagace et al. ..., 252/413 4,419, 195 12/1983 Young ................................... 204/78 9 Claims, 1 Drawing Sheet CUMULATIVE AVERAGE ACETAL CONVERSION, o 9 O 85 75 ALUMNA TYPE -- TYPE A, 49 m2/g -- TYPEB, 53 m2/g 65 -e- TYPE C, < m2/g -- TYPE D, 237 m2/g O 2OO 4OO 6OO 8OO OOO 2OO 4OO 6OO TIME ON STREAM, HRS U.S. -

Transcription 12.01.12
Lecture 2B • 01/12/12 We covered three different reactions for converting alcohols into leaving groups. One was to turn an alcohol into an alkyl chloride, that was using thionyl chloride. Second reaction was using tosyl chloride; the primary difference between those two reactions is one of stereochemistry. An inversion of stereochemistry does occur if you use thionyl chloride, because it does affect the carbon-oxygen bond, but because forming a tosylate does not touch the carbon-oxygen bond, only the oxygen- hydrogen bond, there’s no change in stereochemistry there. We then saw phosphorus tribromide that reacts very similarly to the thionyl chloride; you get an alkyl bromide instead, but it also has inversion of configuration. The last reaction is not a new mechanism, it is just an Sn2 reaction, it’s called the Finkelstein reaction. Really this works, in a sense, off of Le Châtelier’s principle. In solution, in theory, sodium iodide can displace bromide, but sodium bromide can displace iodide, so you can have an Sn2 reaction that goes back and forth and back and forth and back and forth. Except, sodium iodide is somewhat soluble in acetone, while sodium chloride and sodium bromide are not. So, in fact, one of the things that we get out of this as a by-product is sodium bromide, which, again, is not soluble in acetone and therefore precipitates out and is no longer part of the reaction mixture, so there’s no reverse reaction possible. Because of this solubility trick, it allows this reaction to be pulled forward, which means you can get the alkyl iodide. -

Oxo Alcohols Market.Pdf
Oxo Alcohols Market – Business Opportunities, Key-players Analysis and Forecast to 2020 Oxo alcohols are prepared from a two-step process. First step is to convert olefins to alcohols by reaction with syn gas (carbon monoxide & hydrogen) followed by hydrogenation of the resultant aldehyde. In the first step, hydroformylation reaction is carried out under low pressure in the presence of rhodium catalyst (LP Oxo process). For example, ethylene may be hydro formulated to produce normal-butyraldehyde and iso-butyraldehyde which are hydrogenated to produce n-butanol and isobutanol. Browse detailed TOC, Tables, Figures, Charts and Companies mentioned in Oxo alcohols market research report at- http://www.absolutereports.com/10165437 The major applications of Oxo alcohols are plasticizers, paints, coatings & adhesives, lubricant additives, and chemical intermediates. Asia-Pacific is the global leader in the consumption of Oxo alcohols and this dominance is expected to continue till 2020. China is the key market in the region, consuming more than half of the demand for Oxo alcohols, followed by Japan, South Korea, and India, where consumption is growing steadily. Increase in the consumption of Oxo alcohol for air conditioning & refrigeration, chemical processing, transportation, and consumer goods industry has been observed in Asia-Pacific due to continued industrialization and rise in the manufacturing sector of the region. Growing innovation and developments in the Oxo alcohol consumer industries are directly affecting the rise in the use of Oxo alcohols. Request for sample PDF of Oxo alcohols market research report at- http://www.absolutereports.com/enquiry/request-sample/10165437 The Oxo alcohols market is very competitive due to the presence of big players in the business, which inhibits the new entrants and local manufacturers to capture a good share at global level. -

1.1. Introduction the Reaction Between Olefinic Double Bond and a Mixture
Chapter-1 Hydroformylation of alkenes 1.1. Introduction The reaction between olefinic double bond and a mixture of carbon monoxide and hydrogen leads to linear and branched aldehydes as primary products and is known as hydroformylation reaction. Aldehydes O H O (i) Catalyst R R + CO + H + 2 (ii) Pressure H R alkene (iii) Temperature linear (normal) branched (iso) side reactions R R alkene isomerization alkene hydrogenation Adkins [1] has introduced the term “hydroformylation” for this reaction, since there is attack of hydrogen and formyl group (–CHO) at the unsaturated center of the carbon chain of alkenes. This reaction is an ideal example of atom economic C-C bond formation since the product aldehyde is formed with an additional carbon atom than starting olefin. Although the primary products of hydroformylation reactions are aldehydes, the formation of other oxygen containing by-products [2] accompanied with the side-products such as alcohols, formic acid esters, and higher boiling residues is also observed. Other products namely oxo alcohol and carboxylic acid are formed by hydrogenation and oxidation respectively of the primary oxo products, the aldehydes. Otto Roelen discovered hydroformylation reaction in 1938 [3], during his work on increasing the chain-length of Fischer-Tropsch (FT) hydrocarbons. When a mixture of ethylene and syn-gas was passed over a fixed bed cobalt containing catalyst at 1500C and 100 bar pressure, Roelen’s could perceive, isolate and characterize the small amount of propanal (and diethyl ketone) that had formed under the unconventional F-T conditions. It was his scientific perceptions that made him possible to link the formation of propanal from ethylene and syn-gas, which was catalyzed by metal-carbonyl formed the F-T reaction conditions. -

Predicting Distribution of Ethoxylation Homologues With
1 PREDICTING THE DISTRIBUTION OF ETHOXYLATION HOMOLOGUES WITH A PROCESS SIMULATOR Nathan Massey, Chemstations, Inc. Introduction Ethoxylates are generally obtained by additions of ethylene oxide (EO) to compounds containing dissociated protons. Substrates used for ethoxylation are primarily linear and branched C12-C18 alcohols, alkyl phenols, nonyl (propylene trimer) or decyl (propylene tetramer) groups, fatty acids and fatty acid derivatives. The addition of EO to a substrate containing acidic hydrogen is catalyzed by bases or Lewis acids. Amphoteric catalysts, as well as heterogeneous catalysts are also used. The degree of ethoxylation ( the moles of EO added per mole of substrate ) varies over wide ranges, in general between 3 and 40, and is chosen according to the intended use. As an illustration of how this distribution might be predicted using a process simulator, Chemcad was used to simulate the ethoxylation of Nonylphenols. Description of the Ethoxylation Chemistry The reaction mechanisms of base catalyzed and acid catalyzed ethoxylation differ, which affects the composition of the reaction products. In base catalyzed ethoxylation an alcoholate anion, formed initially by reaction with the catalyst ( alkali metal, alkali metal oxide, carbonate, hydroxide, or alkoxide ) nucleophilically attacks EO. The resulting union of the EO addition product can undergo an equilibrium reaction with the alcohol starting material or ethoxylated product, or can react further with EO: Figure 1 O RO- + H2CCH2 - - RO CH2CH2O O ROH H2CCH2 - - RO RO CH2CH2OH RO RO CH2CH2O 2 As Figure 1 illustrates, in alkaline catalyzed ethoxylations several reactions proceed in parallel. The addition of EO to an anion with the formation of an ether bond is irreversible. -

Reactions of Alcohols & Ethers 1
REACTIONS OF ALCOHOLS & ETHERS 1. Combustion (Extreme Oxidation) alcohol + oxygen carbon dioxide + water 2 CH3CH2OH + 6 O2 4 CO2 + 6 H2O 2. Elimination (Dehydration) ° alcohol H 2 S O 4/ 1 0 0 C alkene + water H2SO4/100 ° C CH3CH2CH2OH CH3CH=CH2 + H2O 3. Condensation ° excess alcohol H 2 S O 4 / 1 4 0 C ether + water H2SO4/140 ° C 2 CH3CH2OH CH3CH2OCH2CH3 + H2O 4. Substitution Lucas Reagent alcohol + hydrogen halide Z n C l2 alkyl halide + water ZnCl2 CH3CH2OH + HCl CH3CH2Cl + H2O • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol→ turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol → turns cloudy after 5 minutes • Primary Alcohol → takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate → chromium 3+ (orange) → (green) permanganate → manganese (IV) oxide (purple) → (brown) Tertiary Alcohol not oxidized under normal conditions CH3 KMnO4 H3C C OH NO REACTION K2Cr2O7 CH3 tertbutyl alcohol Secondary Alcohol ketone + hydrogen ions H O KMnO4 H3C C CH3 + K2Cr2O7 C + 2 H H3C CH3 OH propanone 2-propanol Primary Alcohol aldehyde + water carboxylic acid + hydrogen ions O KMnO4 O H H KMnO H H 4 + CH3CH2CH2OH C C C H C C C H + 2 H K2Cr2O7 + H2O K Cr O H H H 2 2 7 HO H H 1-propanol propanal propanoic acid 6. -

Unusual Aldehyde Reductase Activity for Production of Full-Length Fatty Alcohol by Cyanobacterial Aldehyde Deformylating Oxygenase
Unusual Aldehyde Reductase Activity for Production of Full-length Fatty Alcohol by Cyanobacterial Aldehyde Deformylating Oxygenase Supacha Buttranon Vidyasirimedhi Institute of Science and Technology Pattarawan Intasian Vidyasirimedhi Institute of Science and Technology Nidar Treesukkasem Vidyasirimedhi Institute of Science and Technology Juthamas Jaroensuk Vidyasirimedhi Institute of Science and Technology Somchart Maenpuen Burapha university Jeerus Sucharitakul Chulalongkorn University Faculty Of Dentistry Narin Lawan Chiang Mai University Faculty of Science Pimchai Chaiyen Vidyasirimedhi Institute of Science and Technology Thanyaporn Wongnate ( [email protected] ) School of Biomolecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology (VISTEC), 555 Wangchan Valley, Rayong 21210, Thailand https://orcid.org/0000-0001-5072-9738 Research Keywords: Non-heme diiron enzyme, Aldehyde-deformylating oxygenase, NADPH-dependent fatty aldehyde reductase, Fatty alcohol, Oxido-reductase Posted Date: November 5th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-100741/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/28 Abstract Background: Aldehyde-deformylating oxygenase (ADO) is a non-heme di-iron enzyme that catalyzes deformylation of aldehydes to generate alkanes/alkenes. In this study, we report for the rst time that under anaerobic or limited oxygen conditions, Prochlorococcus marinus (PmADO) can generate full- length fatty alcohols from fatty aldehydes without eliminating a carbon unit. Results: Unlike the native activity of ADO which requires electrons from the Fd/FNR electron transfer complex, the aldehyde reduction activity of ADO requires only NADPH. Our results demonstrated that yield of alcohol products can be affected by oxygen concentration and type of aldehyde.