NOT to PO' F^FV U VEO H 4 Iic Libkahv M# Lavestigatioa of Eom
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

SAFETY DATA SHEET Revision Date 28.07.2021 According to Regulation (EC) No
Version 7.0 SAFETY DATA SHEET Revision Date 28.07.2021 according to Regulation (EC) No. 1907/2006 Print Date 02.10.2021 GENERIC EU MSDS - NO COUNTRY SPECIFIC DATA - NO OEL DATA SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifiers Product name : Aluminum iodide Product Number : 208493 Brand : Aldrich REACH No. : A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline. CAS-No. : 7784-23-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances 1.3 Details of the supplier of the safety data sheet Company : Sigma-Aldrich Inc. 3050 SPRUCE ST ST. LOUIS MO 63103 UNITED STATES Telephone : +1 314 771-5765 Fax : +1 800 325-5052 1.4 Emergency telephone Emergency Phone # : 800-424-9300 CHEMTREC (USA) +1-703- 527-3887 CHEMTREC (International) 24 Hours/day; 7 Days/week SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 Skin corrosion (Sub-category 1B), H314 Serious eye damage (Category 1), H318 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2 Label elements Labelling according Regulation (EC) No 1272/2008 Aldrich- 208493 Page 1 of 8 The life science business of Merck operates as MilliporeSigma in the US and Canada Pictogram Signal word Danger Hazard statement(s) H314 Causes severe skin burns and eye damage. -

WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/025175 Al 26 February 2015 (26.02.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09K 5/06 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/GB2014/052580 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 22 August 2014 (22.08.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13 15098.2 23 August 2013 (23.08.2013) GB (84) Designated States (unless otherwise indicated, for every (71) Applicant: SUNAMP LIMITED [GB/GB]; Unit 1, Satel kind of regional protection available): ARIPO (BW, GH, lite Place, Macmerry, Edinburgh EH33 1RY (GB). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (72) Inventors: BISSELL, Andrew John; C/o SunAmp, Unit TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, 1, Satellite Place, Macmerry, Edinburgh EH33 1RY (GB). -

Alphabetical Index of Substances and Articles
ALPHABETICAL INDEX OF SUBSTANCES AND ARTICLES - 355 - NOTES TO THE INDEX 1. This index is an alphabetical list of the substances and articles which are listed in numerical order in the Dangerous Goods List in Chapter 3.2. 2. For the purpose of determining the alphabetical order the following information has been ignored even when it forms part of the proper shipping name: numbers; Greek letters; the abbreviations “sec” and “tert”; and the letters “N” (nitrogen), “n” (normal), “o” (ortho) “m” (meta), “p” (para) and “N.O.S.” (not otherwise specified). 3. The name of a substance or article in block capital letters indicates a proper shipping name. 4. The name of a substance or article in block capital letters followed by the word “see” indicates an alternative proper shipping name or part of a proper shipping name (except for PCBs). 5. An entry in lower case letters followed by the word “see” indicates that the entry is not a proper shipping name; it is a synonym. 6. Where an entry is partly in block capital letters and partly in lower case letters, the latter part is considered not to be part of the proper shipping name. 7. A proper shipping name may be used in the singular or plural, as appropriate, for the purposes of documentation and package marking. - 356 - INDEX Name and description Class UN No. Name and description Class UN No. Accumulators, electric, see 4.3 3292 Acid mixture, nitrating acid, see 8 1796 8 2794 8 2795 Acid mixture, spent, nitrating acid, see 8 1826 8 2800 8 3028 Acraldehyde, inhibited, see 6.1 1092 ACETAL 3 1088 -
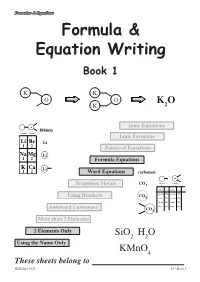
Formula & Equation Writing
Formulae & Equations Formula & Equation Writing Book 1 K K O O K O K 2 Ionic Equations lithium column column 1 2 Ionic Formulae Li Be Li 1 2 Balanced Equations Na Mg Li 1 2 Formula Equations K Ca Li 1 2 Word Equations carbonate valency valency Transition Metals CO3 1 2 name formula name formula ammonium NH4 carbonate CO3 Using Brackets CO 3 cyanide CN chromate CrO4 hydroxide OH sulphate SO4 nitrate NO sulphite SO Awkward Customers 3 3 CO3 More than 2 Elements 2 Elements Only SiO2 H2O Using the Name Only KMnO4 These sheets belong to KHS Sept 2013 page 1 S3 - Book 1 Formulae & Equations What is a Formula ? The formula of a compound tells you two things about the compound :- SiO H O i) which elements are in the compound 2 2 using symbols, and KMnO4 ii) how many atoms of each element are in the compound using numbers. Test Yourself What would be the formula for each of the following? Br H Cl K H C S Al Br O H Cl K H Br Using the Name Only Some compounds have extra information in their carbon monoxide names that allow people to work out and write CO the correct formula. carbon dioxide CO2 The names of the elements appear as usual but dinitrogen tetroxide N O this time the number of each type of atom is 2 4 included using mono- = 1 di- = 12 tri- = 3 tetra- = 4 penta- = 5 hexa- =46 Test Yourself 1 What would be the formula for each of the following? 1. -

Safety Data Sheet Material Name: Fire Extinguisher with Recycled Halon 1301 and Methanol ID: KA006
^^9-^^ Material Safety Data Sheet Material Name: Fire Extinguisher with recycled Halon 1301 and Methanol ID: KA006 I * * * Section 1 - Chemical Product and Company Identification * * * | Chemical Name: Bromotrifluoromethane and Methanol Product Use: Extinguishing Fires Manufacturer Information Kidde Aerospace Phone: 252-246-7004 4200 Airport Drive, NW Wiison, NC 27896 Emergency # 1-800-451-8346; 760-602-8700 (3E Company) I * * * Section 2 - Composition / Information on Ingredients * * * I CAS» Component Percent 75-63-8 bromotrifluoromethane 08 67-56-1 Methyl alcohol 2 Component Related Regulatory Information This product may be regulated, have exposure limits or other information identified as the following: BromofluonDcarbons, Bnamine compounds. Component Information/Information on Non-Hazardous Components This product is considered to be hazardous under 29 CFR 1910.1200 (Hazard Communication). This is a controlled product under the criteria specified in the Canadian Workplace Hazardous Materials Information System (WHMIS). * * * Section 3 - Hazards Identification * * * I Emergency Overview Waming. Asphyxiant. Inhalation of vapors ofthis product may affect the cardiovascular and central nervous system and may cause death. Skin or eye contact with the liquid will cause frostbite. Pressurized container may explode when exposed to heat or flame. May cause blindness. May be harmful ifabsorbed through the skin. Potential Health Effects: Eyes Contacl with the liquid ofthis product will cause frostbite to the eyes. Potential Health Effects: Skin Contact with the liquid ofthis product will cause frostbite to the skin. This product contains methanol which can be absorbed though the skin and cause harmful effects. Potential Health Effects: Ingestion Note likely route of entry. This product contains methanol. Ingestion of methanol may cause irregular heartbeat, headache, dizziness, visual disturbances and blindness. -

Journal-2019-06
INTELLECTUAL PROPERTY REGISTRY OF SAMOA (IPROS) JOURNAL 2019/06 Publication Date – 4 December 2019 GENERAL INFORMATION The Public is hereby notified that the following persons have applied for the Registration of the Trade Marks as set out in Section 50 of the Intellectual Property Act 2011. Any person who wishes to oppose the registration of any of the under-mentioned Trade Marks, must give notice to the Registrar of Trade Marks, c/- Ministry of Commerce, Industry and Labour, Apia, Samoa within three (3) months from the date of this publication. The notice must be in writing and must include a statement of the grounds of opposition. Each notice of opposition must be accompanied by a filing fee of WST$300. Any person who has any doubts as to whether he/she may be affected by the registration of the Trade Mark in Samoa should consult his/her Solicitor immediately. For any enquiries regarding Trade Marks, please contact the Registry of Intellectual Property at the following telephone number (+685) 20441 or email [email protected] Faafetai, Registrar of Intellectual Property Accepted Trade Marks – Direct Filing (NEWSPAPER VERSION) Application No. : WS/T/7350 Representation of Mark Filing Date : 12 September, 2018 Priority Date : N/A Proprietor/Address : NEST CONSTRUCTION COMPANY LTD Vaitele Uta, Samoa Class(es) 37 Application No. : WS/T/7361 Representation of Mark Filing Date : 24 September, 2018 Priority Date : N/A Proprietor/Address : NIKE INNOVATE C.V. One Bowerman Drive, Beaverton, Oregon 97005--6453, United States of America Class(es) 18 and 25 Application No. : WS/T/7464 Representation of Mark Filing Date : 29 July, 2019 Priority Date : 04/03/2019 Proprietor/Address : REIGN BEVERAGE COMPANY LLC 1547 N. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru Et Al
US 2015O175532A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0175532 A1 Mudduluru et al. (43) Pub. Date: Jun. 25, 2015 (54) PROCESS FOR PREPARATION OF (30) Foreign Application Priority Data 2,3-DIHYDROXY BENZONITRILE Jul. 19, 2012 (IN) ........................... 2926/CHFA2012 (71) Applicant: Laurus Labs Private Limited, Hyderabad (IN) Publication Classification (72) Inventors: Hari Krishna Mudduluru, Hyderabad (51) Int. Cl (IN); Shankar Madhavaram, we Hyderabad (IN) C07C 253/30 (2006.01) (52) U.S. Cl. (73) Assignee: Laurus Labs Private Limited, CPC .................................... C07C 253/30 (2013.01) Hyderabad (IN) (21) Appl. No.: 14/415,527 (57)57 ABSTRACT 1-1. The present invention relates to one pot process for the prepa (22) PCT Filed: Jul.18, 2013 ration of 2,3-dihydroxybenzonitrile from 2,3-dialkoxyben (86). PCT No.: PCT/N2O13AOOO447 Zoic acid without prior isolation of the intermediates. Further the invention relates to the preparation of 2,3-dihydroxyben S371 (c)(1), Zonitrile by dealkylation of 2,3-dialkoxybenzonitrile using (2) Date: Jan. 16, 2015 Suitable aluminum salt-amine complex. US 2015/0175532 A1 Jun. 25, 2015 PROCESS FOR PREPARATION OF 0006. Several reviews have been described for deprotec 2,3-DIHYDROXY BENZONITRILE tion of phenolic ethers. For example, phenolic methyl ethers have deprotected to remove the methyl moiety using hydro PRIORITY gen halide Such as hydrogen chloride or hydrogen bromide under highly acidic conditions; highly dark colored products 0001. This application claims the benefit under Indian Provisional Application No. 2926/CHF/2012 filed 19 Jul. formed in these reactions. In addition the phenolic com 2012 and entitled AN IMPROVED PROCESS FOR THE pounds react further with the halogen compounds used thus PREPARATION OF 2,3-DIHYDROXY BENZONITRILE”, setting a major drawback on this route. -

4050 Safety Wash
4050 Safety Wash MG Chemicals UK Limited Version No: A-2.00 Issue Date: 26/03/2019 Safety data sheet according to REACH Regulation (EC) No 1907/2006, as amended by UK REACH Regulations SI 2019/758 Revision Date: 22/04/2021 L.REACH.GB.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking 1.1. Product Identifier Product name 4050 Synonyms SDS Code: 4050-Liquid; 4050-1L, 4050-4L, 4050-20L | UFI: 58R0-10PR-8006-PNCW Other means of identification Safety Wash for Electronics 1.2. Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses Cleaner for electronics that is safe for plastics Uses advised against Not Applicable 1.3. Details of the supplier of the safety data sheet Registered company name MG Chemicals UK Limited MG Chemicals (Head office) Heame House, 23 Bilston Street, Sedgely Dudley DY3 1JA United Address 9347 - 193 Street Surrey V4N 4E7 British Columbia Canada Kingdom Telephone +(44) 1663 362888 +(1) 800-201-8822 Fax Not Available +(1) 800-708-9888 Website Not Available www.mgchemicals.com Email [email protected] [email protected] 1.4. Emergency telephone number Association / Organisation Verisk 3E (Access code: 335388) Emergency telephone +(44) 20 35147487 numbers Other emergency telephone +(0) 800 680 0425 numbers SECTION 2 Hazards identification 2.1. Classification of the substance or mixture Classification according to regulation (EC) No 1272/2008 H225 - Flammable Liquid Category 2, H319 - Eye Irritation Category 2 [CLP] and amendments [1] Legend: 1. Classified by Chemwatch; 2. Classification drawn from Regulation (EU) No 1272/2008 - Annex VI 2.2. -

Chemical Formula of Binary Ionic Compounds – Sheet 1 the Combining Power Or Valency of Silver Is Always 1
Chemical Formula of Binary Ionic Compounds – Sheet 1 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. Binary compound Formula 1 potassium fluoride 26 calcium sulfide 2 calcium chloride 27 lithium bromide 3 barium bromide 28 nickel sulfide 4 silver sulfide 29 zinc phosphide 5 aluminium iodide 30 barium iodide 6 potassium iodide 31 caesium chloride 7 lead(IV) oxide 32 copper bromide 8 zinc nitride 33 sodium nitride 9 silver iodide 34 silver chloride 10 barium fluoride 35 sodium hydride 11 lead(II) iodide 36 potassium nitride 12 silver fluoride 37 cobalt chloride 13 sodium sulfide 38 magnesium sulfide 14 sodium bromide 39 potassium chloride 15 calcium oxide 40 calcium bromide 16 zinc fluoride 41 iron(III) oxide 17 strontium phosphide 42 aluminium fluoride 18 barium sulfide 43 magnesium bromide 19 aluminium oxide 44 iron(III) chloride 20 aluminium chloride 45 barium nitride 21 aluminium sulfide 46 sodium fluoride 22 lead(II) oxide 47 lithium fluoride 23 barium chloride 48 lithium iodide 24 copper chloride 49 lithium hydride 25 barium phosphide 50 potassium oxide “Aluminum” and “cesium” are commonly used alternative spellings for "aluminium" and "caesium that are used in the US. May be freely copied for educational use. ©www.chemicalformula.org Chemical Formula of Binary Ionic Compounds – Sheet 2 The combining power or valency of silver is always 1. All other transition metals are 2 unless otherwise indicated. No. Binary compound Formula No. -

Ep 1831144 B1
(19) & (11) EP 1 831 144 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 37/50 (2006.01) C07C 39/17 (2006.01) 17.08.2011 Bulletin 2011/33 C07C 39/26 (2006.01) (21) Application number: 05816593.7 (86) International application number: PCT/HU2005/000128 (22) Date of filing: 07.12.2005 (87) International publication number: WO 2006/061666 (15.06.2006 Gazette 2006/24) (54) A NEW PROCESS FOR THE PREPARATION OF PHENOLIC HYDROXY-SUBSTITUTED COMPOUNDS NEUES VERFAHREN ZUR HERSTELLUNG PHENOLISCHER HYDROXY SUBSTITUIERTER VERBINDUNGEN PROCEDE D’ELABORATION DE COMPOSES PHENOLIQUES A SUBSTITUTION HYDROXY (84) Designated Contracting States: • BORBÉLY, László AT BE BG CH CY CZ DE DK EE ES FI FR GB GR H-2510 Dorog (HU) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • HAÁSZ, Ferenc SK TR H-2500 Esztergom (HU) Designated Extension States: • JEKKEL, Péter AL BA HR MK YU H-2509 Esztergom-Kertváros (HU) (30) Priority: 08.12.2004 HU 0402530 (74) Representative: HOFFMANN EITLE 11.11.2005 HU 0501044 Patent- und Rechtsanwälte Arabellastraße 4 (43) Date of publication of application: 81925 München (DE) 12.09.2007 Bulletin 2007/37 (56) References cited: (73) Proprietor: Richter Gedeon Nyrt. US-A- 4 339 614 1103 Budapest (HU) • MANABU NODE ET.AL.: "Hard acid and soft (72) Inventors: nucleophile system. 2. Demethylation of methyl • VASS, András ethers of alcohol and phenol with an aluminium H-8200 Veszprém (HU) halide-thiolsystem" J. ORG. CHEM., vol. 45, 1980, • DUDÁS, József pages 4275-4277, XP002379759 cited in the H-8200 Veszprém (HU) application Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Aluminum Bromide (AlBr3) Other means of identification SDS number 1AN Materion Code 1AN CAS number 7727-15-3 Synonyms ALUMINUM BROMIDE * ALUMINUM TRIBROMIDE Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name Materion Advanced Chemicals Inc. Address 407 N 13th Street 1316 W. St. Paul Avenue Milwaukee, WI 53233 United States Telephone 414.212.0257 E-mail [email protected] Contact person Noreen Atkinson Emergency phone number Chemtrec 800.424.9300 2. Hazard(s) identification Physical hazards Corrosive to metals Category 1 Health hazards Acute toxicity, oral Category 3 Skin corrosion/irritation Category 1 Serious eye damage/eye irritation Category 1 Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Toxic if swallowed. May be corrosive to metals. Causes severe skin burns and eye damage. Causes serious eye damage. Precautionary statement Prevention Keep only in original container. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. Wear protective gloves/protective clothing/eye protection/face protection. Response If swallowed: Rinse mouth. Do NOT induce vomiting. If on skin (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. If inhaled: Remove person to fresh air and keep comfortable for breathing. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER or doctor/physician. Specific treatment (see this label). Absorb spillage to prevent material damage. Storage Store locked up. Store in corrosive resistant container with a resistant inner liner.