Phosphoproteomic Investigation of Differential Signalling Downstream of Class IA PI3K Isoforms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

HOLOHAN-DISSERTATION-2015.Pdf (6.419Mb)
IDENTIFYING, CHARACTERIZING AND INHIBITING THE TELOMERASE REGULATORY NETWORK APPROVED BY SUPERVISORY COMMITTEE Jerry Shay, PhD Woodring Wright, MD, PhD David Corey, PhD Rolf Brekken, PhD Melanie Cobb, PhD DEDICATION Dedicated to my parents, Mark and Tracy Holohan, as well as my siblings, Kelly Nudleman and Kyle Holohan for their support and encouragement. IDENTIFYING, CHARACTERIZING AND INHIBITING THE TELOMERASE REGULATORY NETWORK by BRODY CHRISTOPHER HOLOHAN DISSERTATION / THESIS Presented to the Faculty of the Graduate School of Biomedical Sciences The University of Texas Southwestern Medical Center at Dallas In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY The University of Texas Southwestern Medical Center at Dallas Dallas, Texas May, 2015 Copyright by Brody Christopher Holohan, 2015 All Rights Reserved ACKNOWLEDGEMENTS I would like the thank my mentors, Jerry Shay and Woodring Wright for their continuous support, advice and encouragement, as well as allowing me time and resources to pursue my ideas. They are a fantastic team, and I could not have asked for better mentors. I would also like to thank my lab-mates, particularly Dr. Andrew Ludlow, Dr. Yong Zhou, Dr. Tsung-Po Lai, Dr. Oliver Delgado, Dr. Wanil Kim, Dr. Guido Stadler, Kimberly Batten, Ryan Laranger and Neal Jones. I would also like to thank Kevin Kennon for administrative support. Without my collaborators much of this would have been impossible, so I would like to thank Dr. Tim De Meyer, Dr. Abraham Aviv, Dr. Fowzan Aklayura, Dr. Steve Hunt, Dr. Tim Spector, Dr. Massimo Mangino, Dr. Daphne Friedman and Dr. Daniel Eisenberg for their help, intellectual discussions and willingness to pursue challenenging ideas. -

Investigating the Role of Cdk11in Animal Cytokinesis
Investigating the Role of CDK11 in Animal Cytokinesis by Thomas Clifford Panagiotou A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Molecular Genetics University of Toronto © Copyright by Thomas Clifford Panagiotou (2020) Investigating the Role of CDK11 in Animal Cytokinesis Thomas Clifford Panagiotou Master of Science Department of Molecular Genetics University of Toronto 2020 Abstract Finely tuned spatio-temporal regulation of cell division is required for genome stability. Cytokinesis constitutes the final stages of cell division, from chromosome segregation to the physical separation of cells, abscission. Abscission is tightly regulated to ensure it occurs after earlier cytokinetic events, like the maturation of the stem body, the regulatory platform for abscission. Active Aurora B kinase enforces the abscission checkpoint, which blocks abscission until chromosomes have been cleared from the cytokinetic machinery. Currently, it is unclear how this checkpoint is overcome. Here, I demonstrate that the cyclin-dependent kinase CDK11 is required for cytokinesis. Both inhibition and depletion of CDK11 block abscission. Furthermore, the mitosis-specific CDK11p58 kinase localizes to the stem body, where its kinase activity rescues the defects of CDK11 depletion and inhibition. These results suggest a model whereby CDK11p58 antagonizes Aurora B kinase to overcome the abscission checkpoint to allow for successful completion of cytokinesis. ii Acknowledgments I am very grateful for the support of my family and friends throughout my studies. I would also like to express my deep gratitude to Wilde Lab members, both past and present, for their advice and collaboration. In particular, I am very grateful to Matthew Renshaw, whose work comprises part of this thesis. -

In Silico Analysis Identifies Genes Common Between Five Primary Gastrointestinal Cancer Sites with Potential Clinical Applications
ORIGINAL ARTICLE Annals of Gastroenterology (2014) 27, 1-14 In silico analysis identifies genes common between five primary gastrointestinal cancer sites with potential clinical applications Subhankar Chakraborty University of Nebraska Medical Center, Omaha, NE, USA Abstract Background Previous studies have investigated differential gene expression in gastrointes- tinal (GI) epithelial cancers by microarray. The aim of the present study was to use data from the Oncomine database to identify genes that share a similar differential expression in two or more primary GI cancer sites. Methods Five thousand of the most differentially expressed genes in epithelial cancers (com- pared to normal tissue) arising in the pancreas, liver, stomach, esophagus or colorectum were identified (1,000 per primary site) from Oncomine. Using Venn diagrams, genes common to two or more primary GI sites were identified. Functional and pathway analysis was performed on genes that were similarly expressed in ≥3 of the five areas of the GI tract. Results Forty six studies comprising 5,876 samples were included. Overall, 90.6% genes were unique to the respective primary sites, 7.4% shared between two GI primary sites, 1.8% between three and 0.2% between four GI primary sites. Pancreatic and hepatocellular cancers (HCC) shared most number of upregulated genes (N=66) while HCC and gastric cancer shared most downregulated genes (N=59). Genes encoding enzymes comprised the most commonly shared genes between GI primary sites (30.4% of upregulated and 63.2% of downregulated genes). Those genes that were shared between three or more GI primary sites also showed significant differential expression in the same direction in other non-GI cancers. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis
Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis Deepti Verma, Anna-Karin Ekman, Cecilia Bivik Eding and Charlotta Enerbäck The self-archived postprint version of this journal article is available at Linköping University Institutional Repository (DiVA): http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-147791 N.B.: When citing this work, cite the original publication. Verma, D., Ekman, A., Bivik Eding, C., Enerbäck, C., (2018), Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis, Journal of Investigative Dermatology, 138(5), 1088-1093. https://doi.org/10.1016/j.jid.2017.11.036 Original publication available at: https://doi.org/10.1016/j.jid.2017.11.036 Copyright: Elsevier http://www.elsevier.com/ Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis Deepti Verma*a, Anna-Karin Ekman*a, Cecilia Bivik Edinga and Charlotta Enerbäcka *Authors contributed equally aIngrid Asp Psoriasis Research Center, Department of Clinical and Experimental Medicine, Division of Dermatology, Linköping University, Linköping, Sweden Corresponding author: Charlotta Enerbäck Ingrid Asp Psoriasis Research Center, Department of Clinical and Experimental Medicine, Linköping University SE-581 85 Linköping, Sweden Phone: +46 10 103 7429 E-mail: [email protected] Short title Differential methylation in psoriasis Abbreviations CGI, CpG island; DMS, differentially methylated site; RRBS, reduced representation bisulphite sequencing Keywords (max 6) psoriasis, epidermis, methylation, Wnt, susceptibility, expression 1 ABSTRACT Psoriasis is a chronic inflammatory skin disease with both local and systemic components. Genome-wide approaches have identified more than 60 psoriasis-susceptibility loci, but genes are estimated to explain only one third of the heritability in psoriasis, suggesting additional, yet unidentified, sources of heritability. -

Supplementary Table S1. Prioritization of Candidate FPC Susceptibility Genes by Private Heterozygous Ptvs
Supplementary Table S1. Prioritization of candidate FPC susceptibility genes by private heterozygous PTVs Number of private Number of private Number FPC patient heterozygous PTVs in heterozygous PTVs in tumors with somatic FPC susceptibility Hereditary cancer Hereditary Gene FPC kindred BCCS samples mutation DNA repair gene Cancer driver gene gene gene pancreatitis gene ATM 19 1 - Yes Yes Yes Yes - SSPO 12 8 1 - - - - - DNAH14 10 3 - - - - - - CD36 9 3 - - - - - - TET2 9 1 - - Yes - - - MUC16 8 14 - - - - - - DNHD1 7 4 1 - - - - - DNMT3A 7 1 - - Yes - - - PKHD1L1 7 9 - - - - - - DNAH3 6 5 - - - - - - MYH7B 6 1 - - - - - - PKD1L2 6 6 - - - - - - POLN 6 2 - Yes - - - - POLQ 6 7 - Yes - - - - RP1L1 6 6 - - - - - - TTN 6 5 4 - - - - - WDR87 6 7 - - - - - - ABCA13 5 3 1 - - - - - ASXL1 5 1 - - Yes - - - BBS10 5 0 - - - - - - BRCA2 5 6 1 Yes Yes Yes Yes - CENPJ 5 1 - - - - - - CEP290 5 5 - - - - - - CYP3A5 5 2 - - - - - - DNAH12 5 6 - - - - - - DNAH6 5 1 1 - - - - - EPPK1 5 4 - - - - - - ESYT3 5 1 - - - - - - FRAS1 5 4 - - - - - - HGC6.3 5 0 - - - - - - IGFN1 5 5 - - - - - - KCP 5 4 - - - - - - LRRC43 5 0 - - - - - - MCTP2 5 1 - - - - - - MPO 5 1 - - - - - - MUC4 5 5 - - - - - - OBSCN 5 8 2 - - - - - PALB2 5 0 - Yes - Yes Yes - SLCO1B3 5 2 - - - - - - SYT15 5 3 - - - - - - XIRP2 5 3 1 - - - - - ZNF266 5 2 - - - - - - ZNF530 5 1 - - - - - - ACACB 4 1 1 - - - - - ALS2CL 4 2 - - - - - - AMER3 4 0 2 - - - - - ANKRD35 4 4 - - - - - - ATP10B 4 1 - - - - - - ATP8B3 4 6 - - - - - - C10orf95 4 0 - - - - - - C2orf88 4 0 - - - - - - C5orf42 4 2 - - - - -

Thesis FINAL
The Role of CD1d-Mediated Lipid Presentation by Regulatory B Cells in Invariant Natural Killer T Cell Suppression of Autoimmunity by Kristine Oleinika Supervisor Professor Claudia Mauri UCL Immunology I, Kristine Oleinika, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Kristine Oleinika Acknowledgements To Professor Claudia Mauri, my supervisor and mentor Throughout my PhD years I have so admired your energy and passion, which has made me fall in love with science over and over again. Thank you for looking after me, your guidance has been invaluable in my journey in both science and life. I am incredibly grateful for being part of the wonderful team that you have gathered around you, scientific debate that you have fostered in the group, and for encouraging the friendships within it. To all my colleagues You have made this such an adventure, sharing my enthusiasm for science, politics and footwear, with you I have always felt like a member of an incredibly, almost insultingly, over-educated and over-loving family. And I apologise for the many times you have had to endure listening to my idea for “PhD: the musical” and late night immunology aphorisms, or being on the receiving end of the impromptu enforced hugs. Lizzy, Diego and Paul – thank you for making me think about immunology, socialism, feminism, literature and art (..) ad infinitum. And for your helpful comments on the thesis. Madhvi, overachieving mama bear, thanks for being a fantastic listener. -

Telomeres, Aging and Exercise: Guilty by Association?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Federation ResearchOnline COPYRIGHT NOTICE FedUni ResearchOnline https://researchonline.federation.edu.au This is the published version of: Chilton, W., et.al. (2017) Telomeres, aging and exercise: Guilty by association. International Journal of Molecular Sciences, 18(12), p.1-32. Available online at https://doi.org/10.3390/ijms18122573 Copyright © 2017 Chilton, W., et.al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0) (http://creativecommons.org/licenses/by/4.0/). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. International Journal of Molecular Sciences Review Telomeres, Aging and Exercise: Guilty by Association? Warrick Chilton 1,*, Brendan O’Brien 1 and Fadi Charchar 1,2,3,* ID 1 Faculty of Health Sciences and Faculty of Science and Technology, Federation University Australia, Ballarat, VIC 3350, Australia; [email protected] 2 Department of Physiology, University of Melbourne, Melbourne, VIC 3010, Australia 3 Department of Cardiovascular Sciences, University of Leicester, Leicester LE1 7RH, UK * Correspondence: [email protected] (W.C.); [email protected] (F.C.); Tel.: +61-3-5327-6254 (W.C.); +61-3-5327-6098 (F.C.) Received: 7 September 2017; Accepted: 25 November 2017; Published: 29 November 2017 Abstract: Telomeres are repetitive tandem DNA sequences that cap chromosomal ends protecting genomic DNA from enzymatic degradation. -

Structures, Functions, and Mechanisms of Filament Forming Enzymes: a Renaissance of Enzyme Filamentation
Structures, Functions, and Mechanisms of Filament Forming Enzymes: A Renaissance of Enzyme Filamentation A Review By Chad K. Park & Nancy C. Horton Department of Molecular and Cellular Biology University of Arizona Tucson, AZ 85721 N. C. Horton ([email protected], ORCID: 0000-0003-2710-8284) C. K. Park ([email protected], ORCID: 0000-0003-1089-9091) Keywords: Enzyme, Regulation, DNA binding, Nuclease, Run-On Oligomerization, self-association 1 Abstract Filament formation by non-cytoskeletal enzymes has been known for decades, yet only relatively recently has its wide-spread role in enzyme regulation and biology come to be appreciated. This comprehensive review summarizes what is known for each enzyme confirmed to form filamentous structures in vitro, and for the many that are known only to form large self-assemblies within cells. For some enzymes, studies describing both the in vitro filamentous structures and cellular self-assembly formation are also known and described. Special attention is paid to the detailed structures of each type of enzyme filament, as well as the roles the structures play in enzyme regulation and in biology. Where it is known or hypothesized, the advantages conferred by enzyme filamentation are reviewed. Finally, the similarities, differences, and comparison to the SgrAI system are also highlighted. 2 Contents INTRODUCTION…………………………………………………………..4 STRUCTURALLY CHARACTERIZED ENZYME FILAMENTS…….5 Acetyl CoA Carboxylase (ACC)……………………………………………………………………5 Phosphofructokinase (PFK)……………………………………………………………………….6 -
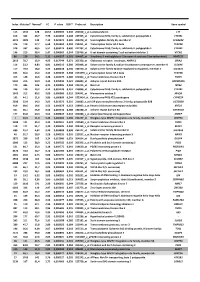
Index Mutated* Normal* FC P Value FDR** Probe Set Description Gene Symbol
Index Mutated* Normal* FC P value FDR** Probe set Description Gene symbol 113 1342 128 10,52 0,000503 0,240 202018_s_at Lactotransferrin LTF 616 362 46,7 7,76 0,003493 0,308 237395_at Cytochrome P450, family 4, subfamily Z, polypeptide 1 CYP4Z1 3073 1009 142 7,10 0,021674 0,385 206378_at Secretoglobin, family 2A, member 2 SCGB2A2 376 119 17,7 6,68 0,001962 0,284 214451_at Transcription factor AP-2 beta TFAP2B 578 337 60,5 5,57 0,003174 0,300 227702_at Cytochrome P450, family 4, subfamily X, polypeptide 1 CYP4X1 146 210 38,4 5,47 0,000669 0,244 219768_at V-set domain containing T cell activation inhibitor 1 VTCN1 86 129 24,2 5,31 0,000327 0,201 204607_at 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (mitochondrial) HMGCS2 2613 78,7 15,9 4,95 0,017744 0,371 205358_at Glutamate receptor, ionotropic, AMPA 2 GRIA2 116 23,2 4,83 4,81 0,000513 0,240 203908_at Solute carrier family 4, sodium bicarbonate cotransporter, member 4 SLC4A4 117 79,4 18,0 4,42 0,000518 0,240 239723_at Solute carrier family 40 (iron-regulated transporter), member 1 SLC40A1 625 56,6 13,0 4,34 0,003549 0,308 1553394_a_atTranscription factor AP-2 beta TFAP2B 413 148 34,6 4,28 0,002175 0,286 240304_s_at Transmembrane channel-like 5 TMC5 5181 216 50,9 4,25 0,039946 0,421 223864_at Ankyrin repeat domain 30A ANKRD30A 179 446 106 4,21 0,000826 0,244 223315_at Netrin 4 NTN4 246 120 29,2 4,12 0,001128 0,251 210096_at Cytochrome P450, family 4, subfamily B, polypeptide 1 CYP4B1 1043 312 80,2 3,89 0,006080 0,319 204041_at Monoamine oxidase B MAOB 192 44,1 11,6 3,80 0,000899 0,244 -

Localization of Glucokinase Gene Expression in the Rat Brain Ronald M
Localization of Glucokinase Gene Expression in the Rat Brain Ronald M. Lynch, Linda S. Tompkins, Heddwen L. Brooks, Ambrose A. Dunn-Meynell, and Barry E. Levin The brain contains a subpopulation of glucosensing neu- rons that alter their firing rate in response to elevated glucose concentrations. In pancreatic -cells, gluco- ammalian feeding behavior and general energy kinase (GK), the rate-limiting enzyme in glycolysis, medi- homeostasis appear to be regulated by circu- ates glucose-induced insulin release by regulating intra- lating levels of nutrients (glucose) and pep- cellular ATP production. A similar role for GK is pro- tides (e.g., leptin, insulin). Sensors to detect lev- posed to underlie neuronal glucosensing. Via in situ M els of these factors have been found to reside within specific hybridization, GK mRNA was localized to hypothalamic areas that are thought to contain relatively large popu- nuclei of the hypothalamus (1–8), where central regulation of lations of glucosensing neurons (the arcuate, ventrome- energy homeostasis is believed to be coordinated. For exam- dial, dorsomedial, and paraventricular nuclei and the lat- ple, large changes in blood glucose are correlated with cen- eral area). GK also was found in brain areas without trally mediated responses such as thermogenesis through known glucosensing neurons (the lateral habenula, the activation of the sympathetic nervous system. These changes bed nucleus stria terminalis, the inferior olive, the are monitored by the brain (9–11), and such responses are retrochiasmatic and medial preoptic areas, and the thal- altered in obesity-prone animals (11–13). Moreover, lesions amic posterior paraventricular, interpeduncular, oculo- of the ventromedial hypothalamus (VMH) prevent the hypo- motor, and anterior olfactory nuclei).