Morphology and Evolution of Scopula, Pseudoscopula and Claw Tufts in Mygalomorphae (Araneae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography of the Caribbean Cyrtognatha Spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J
www.nature.com/scientificreports OPEN Biogeography of the Caribbean Cyrtognatha spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J. Binford3 & Matjaž Kuntner 1,4,5,6 Island systems provide excellent arenas to test evolutionary hypotheses pertaining to gene fow and Received: 23 July 2018 diversifcation of dispersal-limited organisms. Here we focus on an orbweaver spider genus Cyrtognatha Accepted: 1 November 2018 (Tetragnathidae) from the Caribbean, with the aims to reconstruct its evolutionary history, examine Published: xx xx xxxx its biogeographic history in the archipelago, and to estimate the timing and route of Caribbean colonization. Specifcally, we test if Cyrtognatha biogeographic history is consistent with an ancient vicariant scenario (the GAARlandia landbridge hypothesis) or overwater dispersal. We reconstructed a species level phylogeny based on one mitochondrial (COI) and one nuclear (28S) marker. We then used this topology to constrain a time-calibrated mtDNA phylogeny, for subsequent biogeographical analyses in BioGeoBEARS of over 100 originally sampled Cyrtognatha individuals, using models with and without a founder event parameter. Our results suggest a radiation of Caribbean Cyrtognatha, containing 11 to 14 species that are exclusively single island endemics. Although biogeographic reconstructions cannot refute a vicariant origin of the Caribbean clade, possibly an artifact of sparse outgroup availability, they indicate timing of colonization that is much too recent for GAARlandia to have played a role. Instead, an overwater colonization to the Caribbean in mid-Miocene better explains the data. From Hispaniola, Cyrtognatha subsequently dispersed to, and diversifed on, the other islands of the Greater, and Lesser Antilles. Within the constraints of our island system and data, a model that omits the founder event parameter from biogeographic analysis is less suitable than the equivalent model with a founder event. -

Cryptic Species Delimitation in the Southern Appalachian Antrodiaetus
Cryptic species delimitation in the southern Appalachian Antrodiaetus unicolor (Araneae: Antrodiaetidae) species complex using a 3RAD approach Lacie Newton1, James Starrett1, Brent Hendrixson2, Shahan Derkarabetian3, and Jason Bond4 1University of California Davis 2Millsaps College 3Harvard University 4UC Davis May 5, 2020 Abstract Although species delimitation can be highly contentious, the development of reliable methods to accurately ascertain species boundaries is an imperative step in cataloguing and describing Earth's quickly disappearing biodiversity. Spider species delimi- tation remains largely based on morphological characters; however, many mygalomorph spider populations are morphologically indistinguishable from each other yet have considerable molecular divergence. The focus of our study, Antrodiaetus unicolor species complex which contains two sympatric species, exhibits this pattern of relative morphological stasis with considerable genetic divergence across its distribution. A past study using two molecular markers, COI and 28S, revealed that A. unicolor is paraphyletic with respect to A. microunicolor. To better investigate species boundaries in the complex, we implement the cohesion species concept and employ multiple lines of evidence for testing genetic exchangeability and ecological interchange- ability. Our integrative approach includes extensively sampling homologous loci across the genome using a RADseq approach (3RAD), assessing population structure across their geographic range using multiple genetic clustering analyses that include STRUCTURE, PCA, and a recently developed unsupervised machine learning approach (Variational Autoencoder). We eval- uate ecological similarity by using large-scale ecological data for niche-based distribution modeling. Based on our analyses, we conclude that this complex has at least one additional species as well as confirm species delimitations based on previous less comprehensive approaches. -

The Road Travelled by Australian Trapdoor Spiders
Discovered words and photo by Mark Harvey, WA Museum ustralia is home to many unique spiders with most species occurring Anowhere else on Earth. Many have their origins in the distant past, when Australia was part of Gondwana in the Mesozoic, ca. 180 million years ago. Australia, New Zealand, South America, Africa, Madagascar, Antarctica and the Indian sub-continent, plus a few small islands, once formed a massive southern supercontinent known as Gondwana that gradually fragmented from the Jurassic, ca. 180–160 million years ago. Evidence of the connection between these continental blocks can be found in the fossil record The road travelled by Australian of some plants and animals, but most strikingly in the presence of related groups trapdoor spiders of organisms in the modern biota. But back to spiders. There are three major groups of spiders: the Mesothelae (a Australia that lives in shallow burrows with Above An undescribed species of Conothele. group of primitive spiders now only found in a flap-like lid. It was discovered by Adelaide Asia), the Mygalomorphae (trapdoor spiders University PhD student, Sophie Harrison, and their relatives) and the Araneomorphae to be most closely related to spiders of (all other spiders). The Australian the same genus from South Africa. Using timeline of Australia bumping into Asia. mygalomorphs include trapdoor, funnel- molecular sequence data, Sophie found that He also noted that there were two distinct web and mouse spiders, and tarantulas. the spider, Moggridgea rainbowi, diverged habitat preferences for Australian Conothele. Most Australian mygalomorph spiders from its African cousins sometime between Some species built burrows on tree trunks have their origins in Gondwana. -

Prey of the Jumping Spider Phidippus Johnsoni (Araneae : Salticidae)
Jackson, R. R . 1977 . Prey of the jumping spider Phidippus johnsoni (Araneae : Salticidae) . J. Arachnol. 5 :145-149 . PREY OF THE JUMPING SPIDER PHIDIPPUS JOHNSONI (ARANEAE : SALTICIDAE) Robert R. Jackson I Zoology Departmen t University of Californi a Berkeley, California 9472 0 ABSTRACT Field data indicate that P. johnsoni is an euryphagous predator, whose diet includes organisms (aphids, ants, opilionids) sometimes considered distasteful to spiders . Other spiders are preyed upon , including conspecifics. Prey size tends to be one quarter to three quarters the size of the predator . INTRODUCTION Since spiders are probably a dominant group of predators of insects (Bristowe, 1941 ; Riechert, 1974; Turnbull, 1973), there is considerable interest in their feeding ecology . Spiders have usually been considered to be euryphagous predators with a stabilizing , rather than regulative, effect on insect populations (Riechert, 1974) . However, informa- tion concerning the prey taken by particular spider species, in the field, is limited . Field studies by Edgar (1969, 1970), Robinson and Robinson (1970) and Turnbull (1960) are especially noteworthy . During the course of a study of the reproductive biology of Phidippus johnsoni (Peckham and Peckham) (Jackson, 1976), occasionally individuals of this species were found in the field holding prey in their chelicerae . Each prey discovered in this way i s listed in Table 1 . In addition, Ken Evans and Charles Griswold, who were familiar wit h this species, recorded observations of P. johnsoni with prey. (Their data are included in Table 1 .) These data came from a variety of habitats in western North America, most o f which have been described elsewhere (Jackson, 1976) . -

Transcriptome Characterization of the Aptostichus Atomarius Species Complex Nicole L
Garrison et al. BMC Evolutionary Biology (2020) 20:68 https://doi.org/10.1186/s12862-020-01606-7 RESEARCH ARTICLE Open Access Shifting evolutionary sands: transcriptome characterization of the Aptostichus atomarius species complex Nicole L. Garrison1*, Michael S. Brewer2 and Jason E. Bond3 Abstract Background: Mygalomorph spiders represent a diverse, yet understudied lineage for which genomic level data has only recently become accessible through high-throughput genomic and transcriptomic sequencing methods. The Aptostichus atomarius species complex (family Euctenizidae) includes two coastal dune endemic members, each with inland sister species – affording exploration of dune adaptation associated patterns at the transcriptomic level. We apply an RNAseq approach to examine gene family conservation across the species complex and test for patterns of positive selection along branches leading to dune endemic species. Results: An average of ~ 44,000 contigs were assembled for eight spiders representing dune (n = 2), inland (n = 4), and atomarius species complex outgroup taxa (n = 2). Transcriptomes were estimated to be 64% complete on average with 77 spider reference orthologs missing from all taxa. Over 18,000 orthologous gene clusters were identified within the atomarius complex members, > 5000 were detected in all species, and ~ 4700 were shared between species complex members and outgroup Aptostichus species. Gene family analysis with the FUSTr pipeline identified 47 gene families appearing to be under selection in the atomarius ingroup; four of the five top clusters include sequences strongly resembling other arthropod venom peptides. The COATS pipeline identified six gene clusters under positive selection on branches leading to dune species, three of which reflected the preferred species tree. -

Final Project Completion Report
CEPF SMALL GRANT FINAL PROJECT COMPLETION REPORT Organization Legal Name: - Tarantula (Araneae: Theraphosidae) spider diversity, distribution and habitat-use: A study on Protected Area adequacy and Project Title: conservation planning at a landscape level in the Western Ghats of Uttara Kannada district, Karnataka Date of Report: 18 August 2011 Dr. Manju Siliwal Wildlife Information Liaison Development Society Report Author and Contact 9-A, Lal Bahadur Colony, Near Bharathi Colony Information Peelamedu Coimbatore 641004 Tamil Nadu, India CEPF Region: The Western Ghats Region (Sahyadri-Konkan and Malnad-Kodugu Corridors). 2. Strategic Direction: To improve the conservation of globally threatened species of the Western Ghats through systematic conservation planning and action. The present project aimed to improve the conservation status of two globally threatened (Molur et al. 2008b, Siliwal et al., 2008b) ground dwelling theraphosid species, Thrigmopoeus insignis and T. truculentus endemic to the Western Ghats through systematic conservation planning and action. Investment Priority 2.1 Monitor and assess the conservation status of globally threatened species with an emphasis on lesser-known organisms such as reptiles and fish. The present project was focused on an ignored or lesser-known group of spiders called Tarantulas/ Theraphosid spiders and provided valuable information on population status and potential conservation sites in Uttara Kannada district, which will help in future monitoring and assessment of conservation status of the two globally threatened theraphosid species T. insignis and Near Threatened T. truculentus. Investment Priority 2.3. Evaluate the existing protected area network for adequate globally threatened species representation and assess effectiveness of protected area types in biodiversity conservation. -

Miranda ZA 2018.Pdf
Zoologischer Anzeiger 273 (2018) 33–55 Contents lists available at ScienceDirect Zoologischer Anzeiger jou rnal homepage: www.elsevier.com/locate/jcz Review of Trichodamon Mello-Leitão 1935 and phylogenetic ଝ placement of the genus in Phrynichidae (Arachnida, Amblypygi) a,b,c,∗ a Gustavo Silva de Miranda , Adriano Brilhante Kury , a,d Alessandro Ponce de Leão Giupponi a Laboratório de Aracnologia, Museu Nacional do Rio de Janeiro, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista s/n, São Cristóvão, Rio de Janeiro-RJ, CEP 20940-040, Brazil b Entomology Department, National Museum of Natural History, Smithsonian Institution, 10th St. & Constitution Ave NW, Washington, DC, 20560, USA c Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark (Zoological Museum), University of Copenhagen, Universitetsparken 15, 2100, Copenhagen, Denmark d Servic¸ o de Referência Nacional em Vetores das Riquetsioses (LIRN), Colec¸ ão de Artrópodes Vetores Ápteros de Importância em Saúde das Comunidades (CAVAISC), IOC-FIOCRUZ, Manguinhos, 21040360, Rio de Janeiro, RJ, Brazil a r t i c l e i n f o a b s t r a c t Article history: Amblypygi Thorell, 1883 has five families, of which Phrynichidae is one of the most diverse and with a Received 18 October 2017 wide geographic distribution. The genera of this family inhabit mostly Africa, India and Southeast Asia, Received in revised form 27 February 2018 with one genus known from the Neotropics, Trichodamon Mello-Leitão, 1935. Trichodamon has two valid Accepted 28 February 2018 species, T. princeps Mello-Leitão, 1935 and T. froesi Mello-Leitão, 1940 which are found in Brazil, in the Available online 10 March 2018 states of Bahia, Goiás, Minas Gerais and Rio Grande do Norte. -

The Case of Embrik Strand (Arachnida: Araneae) 22-29 Arachnologische Mitteilungen / Arachnology Letters 59: 22-29 Karlsruhe, April 2020
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arachnologische Mitteilungen Jahr/Year: 2020 Band/Volume: 59 Autor(en)/Author(s): Nentwig Wolfgang, Blick Theo, Gloor Daniel, Jäger Peter, Kropf Christian Artikel/Article: How to deal with destroyed type material? The case of Embrik Strand (Arachnida: Araneae) 22-29 Arachnologische Mitteilungen / Arachnology Letters 59: 22-29 Karlsruhe, April 2020 How to deal with destroyed type material? The case of Embrik Strand (Arachnida: Araneae) Wolfgang Nentwig, Theo Blick, Daniel Gloor, Peter Jäger & Christian Kropf doi: 10.30963/aramit5904 Abstract. When the museums of Lübeck, Stuttgart, Tübingen and partly of Wiesbaden were destroyed during World War II between 1942 and 1945, also all or parts of their type material were destroyed, among them types from spider species described by Embrik Strand bet- ween 1906 and 1917. He did not illustrate type material from 181 species and one subspecies and described them only in an insufficient manner. These species were never recollected during more than 110 years and no additional taxonomically relevant information was published in the arachnological literature. It is impossible to recognize them, so we declare these 181 species here as nomina dubia. Four of these species belong to monotypic genera, two of them to a ditypic genus described by Strand in the context of the mentioned species descriptions. Consequently, without including valid species, the five genera Carteroniella Strand, 1907, Eurypelmella Strand, 1907, Theumella Strand, 1906, Thianella Strand, 1907 and Tmeticides Strand, 1907 are here also declared as nomina dubia. Palystes modificus minor Strand, 1906 is a junior synonym of P. -

Phylogenomic Analysis and Revised Classification of Atypoid Mygalomorph Spiders (Araneae, Mygalomorphae), with Notes on Arachnid Ultraconserved Element Loci
Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci Marshal Hedin1, Shahan Derkarabetian1,2,3, Adan Alfaro1, Martín J. Ramírez4 and Jason E. Bond5 1 Department of Biology, San Diego State University, San Diego, CA, United States of America 2 Department of Biology, University of California, Riverside, Riverside, CA, United States of America 3 Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, United States of America 4 Division of Arachnology, Museo Argentino de Ciencias Naturales ``Bernardino Rivadavia'', Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina 5 Department of Entomology and Nematology, University of California, Davis, CA, United States of America ABSTRACT The atypoid mygalomorphs include spiders from three described families that build a diverse array of entrance web constructs, including funnel-and-sheet webs, purse webs, trapdoors, turrets and silken collars. Molecular phylogenetic analyses have generally supported the monophyly of Atypoidea, but prior studies have not sampled all relevant taxa. Here we generated a dataset of ultraconserved element loci for all described atypoid genera, including taxa (Mecicobothrium and Hexurella) key to understanding familial monophyly, divergence times, and patterns of entrance web evolution. We show that the conserved regions of the arachnid UCE probe set target exons, such that it should be possible to combine UCE and transcriptome datasets in arachnids. We also show that different UCE probes sometimes target the same protein, and under the matching parameters used here show that UCE alignments sometimes include non- Submitted 1 February 2019 orthologs. Using multiple curated phylogenomic matrices we recover a monophyletic Accepted 28 March 2019 Published 3 May 2019 Atypoidea, and reveal that the family Mecicobothriidae comprises four separate and divergent lineages. -
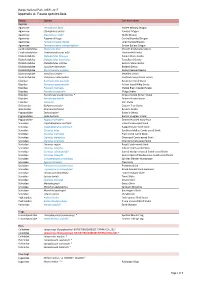
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

Rossi Gf Me Rcla Par.Pdf (1.346Mb)
RESSALVA Atendendo solicitação da autora, o texto completo desta dissertação será disponibilizado somente a partir de 28/02/2021. UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” Instituto de Biociências – Rio Claro Departamento de Zoologia Giullia de Freitas Rossi Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae RIO CLARO – SP Abril/2019 Giullia de Freitas Rossi Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae Dissertação apresentada ao Departamento de Zoologia do Instituto de Biociências de Rio Claro, como requisito para conclusão de Mestrado do Programa de Pós-Graduação em Zoologia. Orientador: Prof. Dr. José Paulo Leite Guadanucci RIO CLARO – SP Abril/2019 Rossi, Giullia de Freitas R832t Taxonomia e biogeografia de aranhas cavernícolas da infraordem Mygalomorphae / Giullia de Freitas Rossi. -- Rio Claro, 2019 348 f. : il., tabs., fotos, mapas Dissertação (mestrado) - Universidade Estadual Paulista (Unesp), Instituto de Biociências, Rio Claro Orientador: José Paulo Leite Guadanucci 1. Aracnídeo. 2. Ordem Araneae. 3. Sistemática. I. Título. Sistema de geração automática de fichas catalográficas da Unesp. Biblioteca do Instituto de Biociências, Rio Claro. Dados fornecidos pelo autor(a). Essa ficha não pode ser modificada. Dedico este trabalho à minha família. AGRADECIMENTOS Agradeço ao meus pais, Érica e José Leandro, ao meu irmão Pedro, minha tia Jerusa e minha avó Beth pelo apoio emocional não só nesses dois anos de mestrado, mas durante toda a minha vida. À José Paulo Leite Guadanucci, que aceitou ser meu orientador, confiou em mim e ensinou tudo o que sei sobre Mygalomorphae. Ao meu grande amigo Roberto Marono, pelos anos de estágio e companheirismo na UNESP Bauru, onde me ensinou sobre aranhas, e ao incentivo em ir adiante. -

Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara Region, Western Australia
Zootaxa 3637 (5): 521–540 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3637.5.2 http://zoobank.org/urn:lsid:zoobank.org:pub:447D8DF5-F922-4B3A-AC43-A85225E56C57 New species of Mouse Spiders (Araneae: Mygalomorphae: Actinopodidae: Missulena) from the Pilbara region, Western Australia DANILO HARMS1, 2, 3, 4 & VOLKER W. FRAMENAU1, 2, 3 1 School of Animal Biology, The University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia. 2 Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. 3 Phoenix Environmental Sciences Pty Ltd, 1/511 Wanneroo Road, Balcatta, Western Australia 6021, Australia. 4 Corresponding author. E-mail: [email protected] Abstract Two new species of Mouse Spiders, genus Missulena, from the Pilbara region in Western Australia are described based on morphological features of males. Missulena faulderi sp. nov. and Missulena langlandsi sp. nov. are currently known from a small area in the southern Pilbara only. Mitochondrial cytochrome c oxidase subunit I (COI) sequence divergence failed in clearly delimiting species in Missulena, but provided a useful, independent line of evidence for taxonomic work in addition to morphology. Key words: taxonomy, systematics, barcoding, mitochondrial DNA, short-range endemism, Actinopus, Plesiolena Introduction The Actinopodidae Simon, 1892 is a small family of mygalomorph spiders with a Gondwanan distribution that includes three genera: Actinopus Perty, 1833 (27 species), Missulena Walckenaer, 1805 (11 species) and Plesiolena Goloboff & Platnick, 1987 (two species). Actinopus and Plesiolena are known only from South and Central America (Platnick 2012).