Comment on the Gas Constant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Ideal Gas Law PV = Nrt R = Universal Gas Constant R = 0.08206 L-Atm R = 8.314 J Mol-K Mol-K Example
Ideal gas law PV = nRT R = universal gas constant R = 0.08206 L-atm R = 8.314 J mol-K mol-K Example: In the reaction of oxygen with carbon to produce carbon dioxide, how many liters of oxygen at 747 mm Hg and 21.0 °C are needed to react with 24.022 g of carbon? CHEM 102 Winter 2011 Practice: In the reaction of oxygen and hydrogen to make water, how many liters of hydrogen at exactly 0 °C and 1 atm are needed to react with 10.0 g of oxygen? 2 CHEM 102 Winter 2011 Combined gas law V decreases with pressure (V α 1/P). V increases with temperature (V α T). V increases with particles (V α n). Combining these ideas: V α n x T P For a given amount of gas (constant number of particles, ninitial = nafter) that changes in volume, temperature, or pressure: Vinitial α ninitial x Tinitial Pinitial Vafter α nafter x Tafter Pafter ninitial = n after Vinitial x Pinitial = Vafter x Pafter Tinitial Tafter 3 CHEM 102 Winter 2011 Example: A sample of gas is pressurized to 1.80 atm and volume of 90.0 cm3. What volume would the gas have if it were pressurized to 4.00 atm without a change in temperature? 4 CHEM 102 Winter 2011 Example: A 2547-mL sample of gas is at 421 °C. What is the new gas temperature if the gas is cooled at constant pressure until it has a volume of 1616 mL? 5 CHEM 102 Winter 2011 Practice: A balloon is filled with helium. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

Neighbor List Collision-Driven Molecular Dynamics Simulation for Nonspherical Hard Particles
Neighbor List Collision-Driven Molecular Dynamics Simulation for Nonspherical Hard Particles. I. Algorithmic Details Aleksandar Donev,1, 2 Salvatore Torquato,1, 2, 3, ∗ and Frank H. Stillinger3 1Program in Applied and Computational Mathematics, Princeton University, Princeton NJ 08544 2Materials Institute, Princeton University, Princeton NJ 08544 3Department of Chemistry, Princeton University, Princeton NJ 08544 Abstract In this first part of a series of two papers, we present in considerable detail a collision-driven molecular dynamics algorithm for a system of nonspherical particles, within a parallelepiped sim- ulation domain, under both periodic or hard-wall boundary conditions. The algorithm extends previous event-driven molecular dynamics algorithms for spheres, and is most efficient when ap- plied to systems of particles with relatively small aspect ratios and with small variations in size. We present a novel partial-update near-neighbor list (NNL) algorithm that is superior to previ- ous algorithms at high densities, without compromising the correctness of the algorithm. This efficiency of the algorithm is further increased for systems of very aspherical particles by using bounding sphere complexes (BSC). These techniques will be useful in any particle-based simula- tion, including Monte Carlo and time-driven molecular dynamics. Additionally, we allow for a nonvanishing rate of deformation of the boundary, which can be used to model macroscopic strain and also alleviate boundary effects for small systems. In the second part of this series of papers we specialize the algorithm to systems of ellipses and ellipsoids and present performance results for our implementation, demonstrating the practical utility of the algorithm. ∗ Electronic address: [email protected] 1 I. -

Equipartition of Energy
Equipartition of Energy The number of degrees of freedom can be defined as the minimum number of independent coordinates, which can specify the configuration of the system completely. (A degree of freedom of a system is a formal description of a parameter that contributes to the state of a physical system.) The position of a rigid body in space is defined by three components of translation and three components of rotation, which means that it has six degrees of freedom. The degree of freedom of a system can be viewed as the minimum number of coordinates required to specify a configuration. Applying this definition, we have: • For a single particle in a plane two coordinates define its location so it has two degrees of freedom; • A single particle in space requires three coordinates so it has three degrees of freedom; • Two particles in space have a combined six degrees of freedom; • If two particles in space are constrained to maintain a constant distance from each other, such as in the case of a diatomic molecule, then the six coordinates must satisfy a single constraint equation defined by the distance formula. This reduces the degree of freedom of the system to five, because the distance formula can be used to solve for the remaining coordinate once the other five are specified. The equipartition theorem relates the temperature of a system with its average energies. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions. -
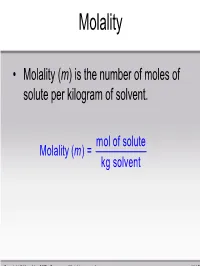
Mol of Solute Molality ( ) = Kg Solvent M
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

Molar Gas Constant
Molar gas constant The molar gas constant (also known as the universal or ideal gas constant and designated by the symbol R ) is an important physical constant which appears in many of the fundamental equations inphysics, chemistry and engineering, such as the ideal gas law, other equations of state and the Nernst equation. R is equivalent to the Boltzmann constant (designated kB) times Avogadro's constant (designated NA) and thus: R = kBNA. Currently the most accurate value as published by the National Institute of Standards and Technology (NIST) is:[1] R = 8.3144621 J · K–1 · mol–1 A number of values of R in other units are provided in the adjacent table. The gas constant occurs in the ideal gas law as follows: PV = nRT or as: PVm = RT where: P is the absolute pressure of the gas T is the absolute temperature of the gas V is the volume the gas occupies n is the number of moles of gas Vm is the molar volume of the gas The specific gas constant The gas constant of a specific gas, as differentiated from the above universal molar gas constant which applies for any ideal gas, is designated by the symbol Rs and is equal to the molar gas constant divided by the molecular mass (M ) of the gas: Rs = R ÷ M The specific gas constant for an ideal gas may also be obtained from the following thermodynamicsrelationship:[2] Rs = cp – cv where cp and cv are the gas's specific heats at constant pressure and constant volume respectively. Some example values of the specific gas constant are: –1 –1 –1 Ammonia (molecular mass of 17.032 g · mol ) : Rs = 0.4882 J · K · g –1 –1 –1 Hydrogen (molecular mass of 2.016 g · mol ) : Rs = 4.1242 J · K · g –1 –1 –1 Methane (molecular mass of 16.043 g · mol ) : Rs = 0.5183 J · K · g Unfortunately, many authors in the technical literature sometimes use R as the specific gas constant without designating it as such or stating that it is the specific gas constant. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Kinetics and Atmospheric Chemistry
12/9/2017 Kinetics and Atmospheric Chemistry Edward Dunlea, Jose-Luis Jimenez Atmospheric Chemistry CHEM-5151/ATOC-5151 Required reading: Finlayson-Pitts and Pitts Chapter 5 Recommended reading: Jacob, Chapter 9 Other reading: Seinfeld and Pandis 3.5 General Outline of Next 3 Lectures • Intro = General introduction – Quick review of thermodynamics • Finlayson-Pitts & Pitts, Chapter 5 A. Fundamental Principles of Gas-Phase Kinetics B. Laboratory Techniques for Determining Absolute Rate Constants for Gas-Phase Reactions C. Laboratory Techniques for Determining Relative Rate Constants for Gas-Phase Reactions D. Reactions in Solution E. Laboratory Techniques for Studying Heterogeneous Reactions F. Compilations of Kinetic Data for Atmospheric Reactions 1 12/9/2017 Kinetics and Atmospheric Chemistry • What we’re doing here… – Photochemistry already covered – We will cover gas phase kinetics and heterogeneous reactions – Introductions to a few techniques used for measuring kinetic parameters • What kind of information do we hope to get out of “atmospheric kinetics”? – Predictive ability over species emitted into atmosphere • Which reactions will actually proceed to products? • Which products will they form? • How long will emitted species remain before they react? Competition with photolysis, wash out, etc. • Pare down list of thousands of possible reactions to the ones that really matter – Aiming towards idea practical predictive abilities • Use look up tables to decide if reaction is likely to proceed and determine an “atmospheric lifetime” -

Chemical Examples for the Fit Equations
Chemical Examples for the Fit Equations The following are just a few of the applications for nonlinear curve fitting. A nice introduction to biological applications of nonlinear curve fitting by Dr. Harvey Motulsky is available on the Web 1. a exp(-bx) f = a e-bx Example: Chemical kinetics first order decay of a reactant. A → P gives [A] = [A] o e-kt a(1-exp(-bx)) f = a (1 - e-bx ) 1.2 1 0.8 0.6 0.4 a(1-exp(-bx)) 0.2 0 0 2 4x 6 8 10 Example: Chemical kinetics first order increase of a product. A → P gives [P] = [P] o(1 - e-kt ) a(1-exp(-b(x-c))) f = a (1 - e-b(x-c) ) Example: This form is the same function as above with an x axis offset. For first order kinetics a delay in the time of the start of the reaction of t o would give the rate law [P] = [P] o(1 - e-k(t-to) ) where t o = c in the fitting function. a(1-exp(-bx)) + c f = a (1 - e-bx ) + c Example: This form introduces a constant offset for first order type growth. For a first order chemical reaction [P] = [P] o(1 - e-kt ) + c where the offset, c, is due to miscalibration or an instrumental artifact. This form can be used interchangeably with f = A e-bx + C as given in the next function with A = -a and C = a+c. a exp(-bx) + c f = a e-bx + c 1 0.8 0.6 0.4 a exp(-bx) + c a exp(-bx) 0.2 0 0 2 4x 6 8 10 Example: Chemical kinetics first order decay of a reactant towards equilibrium.