Atkins' Physical Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phonon Anharmonicity of Ionic Compounds and Metals
Phonon Anharmonicity of Ionic Compounds and Metals Thesis by Chen W. Li In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute of Technology Pasadena, California 2012 (Defended May 4th, 2012) ii © 2012 Chen W. Li All Rights Reserved iii To my lovely wife, Jun, whose support is indispensable through the years, and my son, Derek iv Acknowledgments First and foremost, I would like to thank my advisor, Brent Fultz, for his guidance and support throughout my graduate student years at Caltech, and especially for his efforts to offer me a second chance at admission after the visa difficulty. I am very fortunate to have had him as a mentor and collaborator during these years. Without his insightful advice, this work would not have been possible. I owe many thanks to all current and former members of the Fultz group, who have been a constant source of thoughts and support. In particular, I would like to thank Mike McKerns for his guidance on the Raman and computational work. Special thanks to my officemates, Olivier Delaire (now at Oak Ridge National Lab), Max Kresch (now at IDA), Mike Winterrose (now at MIT Lincoln Lab), and Jorge Muñoz, who made the work much more enjoyable. Thanks to the former DANSE postdocs: Nikolay Markovskiy, Xiaoli Tang, Alexander Dementsov, and J. Brandon Keith. You helped me a lot on programming and calculations. Also thanks to other former group members: Matthew Lucas (now at Air Force Research Laboratory), Rebecca Stevens, Hongjin Tan (now at Contour Energy Systems), and Justin Purewal (now at Ford Motor/University of Michigan); as well as other current group members: Hillary Smith, Tian Lan, Lisa Mauger, Sally Tracy, Nick Stadie, and David Abrecht. -

CHAPTER 12: Thermodynamics Why Chemical Reactions Happen
4/3/2015 CHAPTER 12: Thermodynamics Why Chemical Reactions Happen A tiny fraction of the sun's energy is used to produce complicated, ordered, high- Useful energy is being "degraded" in energy systems such as life the form of unusable heat, light, etc. • Our observation is that natural processes proceed from ordered, high-energy systems to disordered, lower energy states. • In addition, once the energy has been "degraded", it is no longer available to perform useful work. • It may not appear to be so locally (earth), but globally it is true (sun, universe as a whole). 1 4/3/2015 Thermodynamics - quantitative description of the factors that drive chemical reactions, i.e. temperature, enthalpy, entropy, free energy. Answers questions such as- . will two or more substances react when they are mixed under specified conditions? . if a reaction occurs, what energy changes are associated with it? . to what extent does a reaction occur to? Thermodynamics does NOT tell us the RATE of a reaction Chapter Outline . 12.1 Spontaneous Processes . 12.2 Entropy . 12.3 Absolute Entropy and Molecular Structure . 12.4 Applications of the Second Law . 12.5 Calculating Entropy Changes . 12.6 Free Energy . 12.7 Temperature and Spontaneity . 12.8 Coupled Reactions 4 2 4/3/2015 Spontaneous Processes A spontaneous process is one that is capable of proceeding in a given direction without an external driving force • A waterfall runs downhill • A lump of sugar dissolves in a cup of coffee • At 1 atm, water freezes below 0 0C and ice melts above 0 0C • Heat flows -
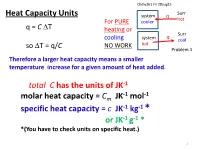
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
![Arxiv:1908.10927V1 [Cond-Mat.Mtrl-Sci] 28 Aug 2019 Calculations, and a Variety of Alloying Elements and Crys- 2 Tal Structures Has Been Considered](https://docslib.b-cdn.net/cover/3340/arxiv-1908-10927v1-cond-mat-mtrl-sci-28-aug-2019-calculations-and-a-variety-of-alloying-elements-and-crys-2-tal-structures-has-been-considered-643340.webp)
Arxiv:1908.10927V1 [Cond-Mat.Mtrl-Sci] 28 Aug 2019 Calculations, and a Variety of Alloying Elements and Crys- 2 Tal Structures Has Been Considered
Impact of anharmonicity on sound wave velocities at extreme conditions Olle Hellman1 and S. I. Simak1 1Department of Physics, Chemistry and Biology (IFM), Link¨opingUniversity, SE-581 83, Link¨oping,Sweden. Theoretical calculations of sound-wave velocities of materials at extreme conditions are of great importance to various fields, in particular geophysics. For example, the seismic data on sound-wave propagation through the solid iron-rich Earth's inner core have been the main source for elucidating its properties and building models. As the laboratory experiments at very high temperatures and pressures are non-trivial, ab initio predictions are invaluable. The latter, however, tend to disagree with experiment. We notice that many attempts to calculate sound-wave velocities of matter at extreme conditions in the framework of quantum-mechanics based methods have not been taking into account the effect of anharmonic atomic vibrations. We show how anharmonic effects can be incorporated into ab initio calculations and demonstrate that in particular they might be non- negligible for iron in Earth's core. Therefore, we open an avenue to reconcile experiment and ab initio theory. Sound-wave velocities of materials at extreme condi- mological values are well constrained, the difference be- tions are important parameters for building models in tween the observations and theory suggests that a simple different physical areas, in particular in geophysics. For model for the inner core based on the commonly assumed example, modeling of Earth's inner core has to rely on phases is wrong. Different and rather complex explana- sound-velocities from seismic data. They, together with tions of this puzzling result have been suggested, includ- other cosmochemical and geochemical data, indicate that ing the partial melting of the inner core.[13] It has been, the inner core should be made out mostly of iron with however, noticed by Voˇcadlo et al.[13] that the disagree- approximately 10 at. -

Unit 4 Vibrational Spectra of Diamotic Molecules
UNIT 4 VIBRATIONAL SPECTRA OF DIAMOTIC MOLECULES Structure 4.1 Introduction Objectives 4.2 Harmonic Oscillator Hooke's Law Equation of Motion Expressions for Force Constant and Characteristic Frequency Potential Energy Cume Quant~sationand Energy Levels 4.3 Diatomic Molecule as Harmonic Oscillator Zero Point Energy Infrared Spectra and Selection Rules Evaluation of Force Constant and Maximum Displacement Isotope Effect Vibrational Term Value 4.4 Anharmonicity Morse Potential Energy Levels of Anharmonic Oscillator and Selection Rules Evaluation of Anharmonicity Constants 4.5 The Vibrating Rotator Energy Levels The IR Spectra and P.Q.R Branches Symmetric Top Vibrating Rotator Model 4.6 Summary 4.7 Terminal Questions 4.8 Answers 4.1 INTRODUCTION While going through Unit 7 of the course "Atoms and Molec,uIes" (CHE-Ol), you might have appreciated the use of vibrational spectroscopy as an analytical technique for the determination of molecular structure. In the last block of this course, two units viz. Units 1 and 3 have been devoted to atomic spectra and rotational spectra, respectively. In this unit and Unit 5, we will discuss vibrational spectroscopy which is another kind of spectroscopy dealing with molecules. In this unit, the theory and applications of vibrational spectra of diatomic molecules will be described. The vibrational spectra of polyatomic molecules is discussed in Unit 5. In this unit, we will start our di.scussion with the classical example of the vibration of a single particle supported by a spring. The similarity of the vibration in a diatomic molecule with the vibration of a single particle is then brought about and possible transitions for the harmonic os;cillator model of diatomic molecules are discussed. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

Neighbor List Collision-Driven Molecular Dynamics Simulation for Nonspherical Hard Particles
Neighbor List Collision-Driven Molecular Dynamics Simulation for Nonspherical Hard Particles. I. Algorithmic Details Aleksandar Donev,1, 2 Salvatore Torquato,1, 2, 3, ∗ and Frank H. Stillinger3 1Program in Applied and Computational Mathematics, Princeton University, Princeton NJ 08544 2Materials Institute, Princeton University, Princeton NJ 08544 3Department of Chemistry, Princeton University, Princeton NJ 08544 Abstract In this first part of a series of two papers, we present in considerable detail a collision-driven molecular dynamics algorithm for a system of nonspherical particles, within a parallelepiped sim- ulation domain, under both periodic or hard-wall boundary conditions. The algorithm extends previous event-driven molecular dynamics algorithms for spheres, and is most efficient when ap- plied to systems of particles with relatively small aspect ratios and with small variations in size. We present a novel partial-update near-neighbor list (NNL) algorithm that is superior to previ- ous algorithms at high densities, without compromising the correctness of the algorithm. This efficiency of the algorithm is further increased for systems of very aspherical particles by using bounding sphere complexes (BSC). These techniques will be useful in any particle-based simula- tion, including Monte Carlo and time-driven molecular dynamics. Additionally, we allow for a nonvanishing rate of deformation of the boundary, which can be used to model macroscopic strain and also alleviate boundary effects for small systems. In the second part of this series of papers we specialize the algorithm to systems of ellipses and ellipsoids and present performance results for our implementation, demonstrating the practical utility of the algorithm. ∗ Electronic address: [email protected] 1 I. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Equipartition of Energy
Equipartition of Energy The number of degrees of freedom can be defined as the minimum number of independent coordinates, which can specify the configuration of the system completely. (A degree of freedom of a system is a formal description of a parameter that contributes to the state of a physical system.) The position of a rigid body in space is defined by three components of translation and three components of rotation, which means that it has six degrees of freedom. The degree of freedom of a system can be viewed as the minimum number of coordinates required to specify a configuration. Applying this definition, we have: • For a single particle in a plane two coordinates define its location so it has two degrees of freedom; • A single particle in space requires three coordinates so it has three degrees of freedom; • Two particles in space have a combined six degrees of freedom; • If two particles in space are constrained to maintain a constant distance from each other, such as in the case of a diatomic molecule, then the six coordinates must satisfy a single constraint equation defined by the distance formula. This reduces the degree of freedom of the system to five, because the distance formula can be used to solve for the remaining coordinate once the other five are specified. The equipartition theorem relates the temperature of a system with its average energies. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in the translational motion of a molecule should equal that of its rotational motions. -

Entropy and Free Energy
Spontaneous Change: Entropy and Free Energy 2nd and 3rd Laws of Thermodynamics Problem Set: Chapter 20 questions 29, 33, 39, 41, 43, 45, 49, 51, 60, 63, 68, 75 The second law of thermodynamics looks mathematically simple but it has so many subtle and complex implications that it makes most chemistry majors sweat a lot before (and after) they graduate. Fortunately its practical, down-to-earth applications are easy and crystal clear. We can build on those to get to very sophisticated conclusions about the behavior of material substances and objects in our lives. Frank L. Lambert Experimental Observations that Led to the Formulation of the 2nd Law 1) It is impossible by a cycle process to take heat from the hot system and convert it into work without at the same time transferring some heat to cold surroundings. In the other words, the efficiency of an engine cannot be 100%. (Lord Kelvin) 2) It is impossible to transfer heat from a cold system to a hot surroundings without converting a certain amount of work into additional heat of surroundings. In the other words, a refrigerator releases more heat to surroundings than it takes from the system. (Clausius) Note 1: Even though the need to describe an engine and a refrigerator resulted in formulating the 2nd Law of Thermodynamics, this law is universal (similarly to the 1st Law) and applicable to all processes. Note 2: To use the Laws of Thermodynamics we need to understand what the system and surroundings are. Universe = System + Surroundings universe Matter Surroundings (Huge) Work System Matter, Heat, Work Heat 1. -

Fundamental Frequency
Dr.Abdulhadi Kadhim. Spectroscopy Ch.3 اﻟﺠﺎﻣﻌﺔ اﻟﺘﻜﻨﻮﻟﻮﺟﯿﺔ ﻗﺴﻢ اﻟﻌﻠﻮم اﻟﺘﻄﺒﯿﻘﯿﺔ اﺳﺘﺎذ اﻟﻤﺎدة :- اﻟﺪﻛﺘﻮر ﻋﺒﺪ اﻟﮭﺎدي ﻛﺎﻇﻢ ﻓﺮع اﻟﻔﯿﺰﯾﺎء اﻟﺘﻄﺒﯿﻘﯿﺔ اﻟﻤﺮﺣﻠﺔ اﻟﺮاﺑﻌﺔ Chapter three: Infrared Spectroscopy (IR) 42 Dr.Abdulhadi Kadhim. Spectroscopy Ch.3 Infrared Spectroscopy (IR) Fundamental region (2.5-15.4mm) IR Near IR (0.75-2.5mm) Far IR (15.4-microwave) Vibrational energy of diatomic molecule:- We are all familiar with the vertical oscillations of amass (m) connected to a stretched spring of a force constant k whose ether end is fixed. The simple harmonic motion with a fundamental frequency:- = ° If two masses in a diatomic molecule m1 and m2 we used the reduced mass \ = in quantum mechanically, the vibrational energy is given by = + υ =0,1,2,3 −−−− ° ° Where υ is the vibrational quantum No. The energy in Cm-1 = =( + ) ° =( + ) \ ° -1 Where ° the freq. in cm . These energy levels are equally spaced \ and the energy of lowest state = ∶ ° ° 43 Dr.Abdulhadi Kadhim. Spectroscopy Ch.3 An harmonic oscillator In addition to rotational motion , molecule has vibrational motion which is an harmonic. Hence a further correction to the centrifugal distortion to be made to account for the vibrational excitation of the molecule. The empirical equation of the potential energy of the diatomic molecule is given by ( ) V =D 1−e This equation called morse equation. V =morse potential. This potential represent the actual curve to a very good degree of approximation except when r=0 ® Vm gives a high finite value while the actual value is infinity. v At r® ∞ ® Vm®De De: dissociation energy. v At r=re ® Vm®0 V D re r 44 Dr.Abdulhadi Kadhim.