Experimental Determination of Heat Capacities and Their Correlation with Theoretical Predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
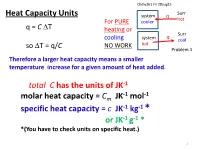
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 Mpa
Volume 96, Number 6, November-December 1991 Journal of Research of the National Institute of Standards and Technology [J. Res. Nat!. Inst. Stand. Technol. 96, 725 (1991)] Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa Volume 96 Number 6 November-December 1991 J. W. Magee Molar heat capacities at constant vol- the Cv (fi,T) data and also show satis- ume (C,) for nitrogen have been mea- factory agreement with published heat National Institute of Standards sured with an automated adiabatic capacity data. The overall uncertainty and Technology, calorimeter. The temperatures ranged of the Cy values ranges from 2% in the Boulder, CO 80303 from 65 to 300 K, while pressures were vapor to 0.5% in the liquid. as high as 35 MPa. Calorimetric data were obtained for a total of 276 state Key words: calorimeter; heat capacity; conditions on 14 isochores. Extensive high pressure; isochorlc; liquid; mea- results which were obtained in the satu- surement; nitrogen; saturation; vapor. rated liquid region (C^^^' and C„) demonstrate the internal consistency of Accepted: August 30, 1991 Glossary ticular, the heat capacity at constant volume {Cv) is a,b,c,d,e Coefficients for Eq. (7) related to P(j^T) by: c. Molar heat capacity at constant volume a Molar heat capacity in the ideal gas state (1) C„<2> Molar heat capacity of a two-phase sample Jo a Molar heat capacity of a saturated liquid sample where C? is the ideal gas heat capacity. Conse- Vbamb Volume of the calorimeter containing sample quently, Cv measurements which cover a wide Vr, Molar volume, dm' • mol"' range of {P, p, T) states are beneficial to the devel- P Pressure, MPa opment of an accurate equation of state for the P. -

HEAT CAPACITY of MINERALS: a HANDS-ON INTRODUCTION to CHEMICAL THERMODYNAMICS David G
HEAT CAPACITY OF MINERALS: A HANDS-ON INTRODUCTION TO CHEMICAL THERMODYNAMICS David G. Bailey Hamilton College Clinton, NY 13323 email:[email protected] INTRODUCTION Minerals are inorganic chemical compounds with a wide range of physical and chemical properties. Geologists frequently measure and observe properties such as hardness, specific gravity, color, etc. Unfortunately, students usually view these properties simply as tools for identifying unknown mineral specimens. One of the objectives of this exercise is to make students aware of the fact that minerals have many additional properties that can be measured, and that all of the physical and chemical properties of minerals have important applications beyond that of simple mineral identification. ROCKS AS CHEMICAL SYSTEMS In order to understand fully many geological processes, the rocks and the minerals of which they are composed must be viewed as complex chemical systems. As with any natural system, minerals and rocks tend toward the lowest possible energy configuration. In order to assess whether or not certain minerals are stable, or if the various minerals in the rock are in equilibrium, one needs to know how much energy exists in the system, and how it is partitioned amongst the various phases. The energy in a system that is available for driving chemical reactions is referred to as the Gibbs Free Energy (GFE). For any chemical reaction, the reactants and the products are in equilibrium (i.e., they all are stable and exist in the system) if the GFE of the reactants is equal to that of the products. For example: reactants 12roducts CaC03 + Si02 CaSi03 + CO2 calcite + quartz wollastonite + carbon dioxide at equilibrium: (Gcalcite+ GqtJ = (Gwoll + GC02) or: ilG = (Greactants- Gproducts)= 0 If the free energy of the reactants and products are not equal, the reaction will proceed to the assemblage with the lowest total energy. -

Physical Chemistry-Ii
____________________________________________________________________________________________________ Subject CHEMISTRY Paper No and Title 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No and 13: Molar heat capacities and Electronic Partition Title function Module Tag CHE_P6_M13 Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Molar Heat Capacities – Solids 3.1 Einstein Theory of Heat Capacity of Solids 4. Partition function – Internal Structure of Atom 4.1 Electronic Partition Function 5. Summary Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to Learn about molar heat capacities of solids Learn about Einstein theory of heat capacities of solids Derive electronic partition function 2. Introduction In our previous module, we did talk about molar heat capacity of gases. Molar heat capacities provide a testing ground for verifying the theoretical -

Equipartition Theorem - Wikipedia 1 of 32
Equipartition theorem - Wikipedia 1 of 32 Equipartition theorem In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in translational motion of a molecule should equal that in rotational motion. The equipartition theorem makes quantitative Thermal motion of an α-helical predictions. Like the virial theorem, it gives the total peptide. The jittery motion is random average kinetic and potential energies for a system at a and complex, and the energy of any given temperature, from which the system's heat particular atom can fluctuate wildly. capacity can be computed. However, equipartition also Nevertheless, the equipartition theorem allows the average kinetic gives the average values of individual components of the energy of each atom to be energy, such as the kinetic energy of a particular particle computed, as well as the average or the potential energy of a single spring. For example, potential energies of many it predicts that every atom in a monatomic ideal gas has vibrational modes. The grey, red an average kinetic energy of (3/2)kBT in thermal and blue spheres represent atoms of carbon, oxygen and nitrogen, equilibrium, where kB is the Boltzmann constant and T respectively; the smaller white is the (thermodynamic) temperature. More generally, spheres represent atoms of equipartition can be applied to any classical system in hydrogen. -

Heats of Transition, Heats of Reaction, Specific Heats, And
Heats of Transition, Heats of Reaction, Specific Heats, and Hess's Law GOAL AND OVERVIEW A simple calorimeter will be made and calibrated. It will be used to determine the heat of fusion of ice, the specific heat of metals, and the heat of several chemical reactions. These heats of reaction will be used with Hess's law to determine another desired heat of reaction. Objectives and Science Skills • Understand, explain, and apply the concepts and uses of calorimetry. • Describe heat transfer processes quantitatively and qualitatively, including those related to heat capacity (including standard and molar), heat (enthalpy) of fusion, and heats (enthalpies) of chemical reactions. • Apply Hess's law using experimental data to determine an unknown heat (enthalpy) of reac- tion. • Quantitatively and qualitatively compare experimental results with theoretical values. • Identify and discuss factors or effects that may contribute to deviations between theoretical and experimental results and formulate optimization strategies. SUGGESTED REVIEW AND EXTERNAL READING • See reference section; textbook information on calorimetry and thermochemistry BACKGROUND The internal energy, E, of a system is not always a convenient property to work with when studying processes occurring at constant pressure. This would include many biological processes and chemical reactions done in lab. A more convenient property is the sum of the internal energy and pressure-volume work (−wsys = P ∆V at constant P). This is called the enthalpy of the system and is given the symbol H. H ≡ E + pV (definition of enthalpy; H) (1) Enthalpy is simply a corrected energy that reflects the change in energy of the system while it is allowed to expand or contract against a constant pressure. -
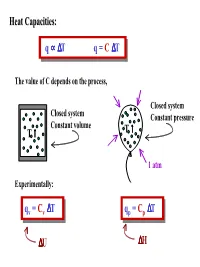
Heat Capacities: Q ∝ ∆T Q = C ∆T T ↑ ∆U T ↑ ∆H Q = C V ∆T Q = C V
Heat Capacities: q ∝∝∆∆Tq =C ∆T The value of C depends on the process, Closed system Closed system Constant pressure Constant volume ↑ T ↑ T 1 atm Experimentally: q = C ∆∆T q = C ∆∆T qvv =Cvv T qpp =Cpp T ∆U ∆H Heat capacity of an “ideal” gas: ClassicallyClassically heat heat energy energy can can be be stored stored in in a a gas gas as as internal internal energy energy with: with: ROTATION ½ kT per degree of freedom kT per degree of freedom VIBRATION TRANSLATION ½ kT per degree of freedom WhenWhen quantization quantization of of energy energy levels levels is is taken taken into into account, account, contributions contributions to to the the heat capacity can be considered classically only if E << kT. Energy levels heat capacity can be considered classically only if Enn << kT. Energy levels with E ≥≥kT contribute little, if at all, to the heat capacity. with Enn kT contribute little, if at all, to the heat capacity. Since vibrational energies are generally comparable to or greater than kT, only rotations and translations store much energy under normal conditions. Classical internal energy of an “ideal” gas: ATOMS: 3 Translations per atom ATOMS: # of moles × U = n 3 RT U = N(3 ½ kT) 2 # of atoms (or molecules) LINEARLINEAR MOLECULES: MOLECULES: 3 Translations per molecule 2 Rotations per molecule U = N 3 kT + N(2 × ½kT) U = n 5 RT 2 2 NON-LINEARNON-LINEAR MOLECULES: MOLECULES: 3 Translations per molecule 3 Rotations per molecule U = N 3 kT + N(3 × ½kT) 2 U = n 3RT Molar heat capacity of an “ideal” gas: q = C ∆T ∆ p p qv = Cv T PV = nRT ∆H = C ∆T ∆U = C ∆T p v P∆V = nR∆T ∆U + P∆V = C ∆T ∆ ∆ p Cv = U/ T ∆U + R∆T = C ∆T 1 mole p Constant Volume ∆ ∆ Cp = U/ T + R Constant Pressure 1.1. -

LEP 3.2.02 Heat Capacity of Gases
R LEP Heat capacity of gases 3.2.02 Related topics Scissors, straight, l 140 mm 64625.00 1 Equation of state for ideal gases, 1st law of thermodynamics, Push-button switch 06039.00 1 universal gas constant, degree of freedom, mole volumes, iso- Connection box 06030.23 1 bars, isotherms, isochors and adiabatic changes of state. PEK carbon resistor 1 W 5 % 1 kOhm 39104.19 1 PEK electrol.capacitor 2 mmF/ 250 V 39105.29 1 Connecting cord, 500 mm, yellow 07361.02 3 Principle and task Connecting cord, 500 mm, red 07361.01 1 Heat is added to a gas in a glass vessel by an electric heater Connecting cord, 750 mm, red 07362.01 1 which is switched on briefly. The temperature increase results Connecting cord, 500 mm, blue 07361.04 4 in a pressure increase, which is measured with a manometer. Tripod base -PASS- 02002.55 1 Under isobaric conditions a temperature increase results in a Retort stand, h 750 mm 37694.00 2 volume dilatation, which can be read from a gas syringe. The Universal clamp 37715.00 2 molar heat capacities CV and Cp are calculated from the pres- Right angle clamp 37697.00 2 sure or volume change. Problems Equipment Determine the molar heat capacities of air at constant volume Precision manometer 03091.00 1 CV and at constant pressure Cp. Barometer/Manometer, hand-held 07136.00 1 Digital counter, 4 decades 13600.93 1 Digital multimeter 07134.00 2 Set-up and procedure Aspirator bottle, clear gl. 1000 ml 34175.00 1 Perform the experimental set-up according to Figs. -

A New Perspective for Kinetic Theory and Heat Capacity
Volume 13 (2017) PROGRESS IN PHYSICS Issue 3 (July) A New Perspective for Kinetic Theory and Heat Capacity Kent W. Mayhew 68 Pineglen Cres., Ottawa, Ontario, K2G 0G8, Canada. E-mail: [email protected] The currently accepted kinetic theory considers that a gas’ kinetic energy is purely trans- lational and then applies equipartition/degrees of freedom. In order for accepted theory to match known empirical finding, numerous exceptions have been proposed. By re- defining the gas’ kinetic energy as translational plus rotational, an alternative explana- tion for kinetic theory is obtained, resulting in a theory that is a better fit with empirical findings. Moreover, exceptions are no longer required to explain known heat capacities. Other plausible implications are discussed. 1 Introduction Various explanations for equipartition’s failure in describ- The conceptualization of a gaseous system’s kinematics orig- ing heat capacities have been proposed. Boltzmann suggested inated in the writings of the 19th century greats. In 1875, that the gases might not be in thermal equilibrium [8]. Planck Maxwell [1] expressed surprise at the ratio of energies (trans- [9] followed by Einstein and Stern [10] argued the possibility lational, rotational and/or vibrational) all being equal. Boltz- of zero-point harmonic oscillator. More recently Dahl [11] mann’s work on statistical ensembles reinforced the current has shown that a zero point oscillator to be illusionary. Lord acceptance of law of equipartition with a gas’s energy being Kelvin [12–13] realized that equipartition maybe wrongly de- equally distributed among all of its degrees of freedom [2–3]. rived. The debate was somewhat ended by Einstein claiming The net result being that the accepted mean energy for each that equipartition’s failure demonstrated the need for quantum independent quadratic term being kT=2. -

Specific Heat of Linear Dense Phase of Nitrogen Atoms Physisorbed
THE HEAT CAPACITY OF NITROGEN CHAINS IN GROOVES OF SINGLE-WALLED CARBON NANOTUBE BUNDLES M.I. Bagatskii, M.S. Barabashko, V.V. Sumarokov B. Verkin Institute for Low Temperature Physics and Engineering, 47 Lenin Ave., 61103 Kharkov, Ukraine e-mail: [email protected] Abstract The heat capacity of bundles of closed-cap single-walled carbon nanotubes with one-dimensional chains of nitrogen molecules adsorbed in the grooves has been first experimentally studied at temperatures from 2 K to 40 K using an adiabatic calorimeter. The contribution of nitrogen CN2 to the total heat capacity has been separated. In the region 2 8 К the behavior of the curve CN2(T) is qualitatively similar to the theoretical prediction of the phonon heat capacity of 1D chains of Kr atoms localized in the grooves of SWNT bundles. Below 3 К the dependence CN2(Т) is linear. Above 8 К the dependence CN2(Т) becomes steeper in comparison with the case of Kr atoms. This behavior of the heat capacity CN2(Т) is due to the contribution of the rotational degrees of freedom of the N2 molecules. PACS: 65.40.Ba Heat capacity; 65.80.–g Thermal properties of small particles, nanocrystals, nanotubes, and other related systems; 68.43.Pq Adsorbate vibrations; 68.65.-k Low-dimensional, mesoscopic, nanoscale and other related systems: structure and nonelectronic properties; 81.07.De Nanotubes; Keywords: heat capacity, single-walled carbon nanotubes bundles, one- dimensional periodic phase of nitrogen molecules. 1 1. Introduction Since the discovery of carbon nanotubes in 1991 [1], investigations of the physical properties of these novel materials have been rated as a fundamentally important trend in physics of condensed matter [2,3].