Determination of Heat Capacities at Constant Volume From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
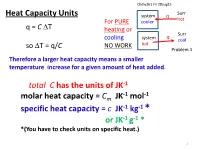
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

REFPROP Documentation Release 10.0
REFPROP Documentation Release 10.0 EWL, IHB, MH, MML May 21, 2018 CONTENTS 1 REFPROP Graphical User Interface3 1.1 General Information...........................................3 1.2 Menu Commands.............................................6 1.3 DLLs................................................... 26 2 REFPROP DLL documentation 27 2.1 High-Level API............................................. 27 2.2 Legacy API................................................ 55 i ii REFPROP Documentation, Release 10.0 REFPROP is an acronym for REFerence fluid PROPerties. This program, developed by the National Institute of Standards and Technology (NIST), calculates the thermodynamic and transport properties of industrially important fluids and their mixtures. These properties can be displayed in Tables and Plots through the graphical user interface; they are also accessible through spreadsheets or user-written applications accessing the REFPROP dll. REFPROP is based on the most accurate pure fluid and mixture models currently available. It implements three models for the thermodynamic properties of pure fluids: equations of state explicit in Helmholtz energy, the modified Benedict-Webb-Rubin equation of state, and an extended corresponding states (ECS) model. Mixture calculations employ a model that applies mixing rules to the Helmholtz energy of the mixture components; it uses a departure function to account for the departure from ideal mixing. Viscosity and thermal conductivity are modeled with either fluid-specific correlations, an ECS method, or in some cases the friction theory method. CONTENTS 1 REFPROP Documentation, Release 10.0 2 CONTENTS CHAPTER ONE REFPROP GRAPHICAL USER INTERFACE 1.1 General Information 1.1.1 About REFPROP REFPROP is an acronym for REFerence fluid PROPerties. This program, developed by the National Institute of Standards and Technology (NIST), calculates the thermodynamic and transport properties of industrially important fluids and their mixtures. -

Physical Chemistry Laboratory, I CHEM 445 Experiment 5 Heat Capacity Ratio for Gases (Revised, 1/10/03)
1 Physical Chemistry Laboratory, I CHEM 445 Experiment 5 Heat Capacity Ratio for Gases (Revised, 1/10/03) The heat capacity for a substance is the amount of heat needed to raise the temperature of the substance by 1 oC or 1 K. The heat capacities depend on the chemical nature of the substance, on the physical state of the substance, on the temperature, and on whether P or V is held constant during the process: CP or CV. For liquids and solids, CV ≈ CP and the two terms are often used interchangeably. However, for ideal gases, it is well known (Tinoco, Jr., Sauer, and Wang or Noggle) that − − CP = CV + R (1) − In this equation, C = molar heat capacity of the gas, either at constant pressure or at constant volume. The bar over a symbol refers to that quantity per mol of substance. The molar heat capacities are defined by the equations, − − − ∂ E − ∂ H CV = and CP = (2) ∂T ∂T V P For a monatomic gas, there is no rotational or vibrational energy and if the temperature is relatively low, only the ground electronic state of the species is populated to any extent. Consequently, only translational energy is involved; and from kinetic theory, − 3R − 5R CV = and CP = (3) 2 2 For polyatomic gases, there are additional forms of energy that contribute to the heat capacity. − − − − E = E(trans) + E(rot) + E(vib) (4) For many simple diatomic molecules, however, the vibrational contribution to the heat 5R capacity is small, and in the neighborhood of room temperature,C = . However, the V 2 heat capacities of diatomic molecules increase with increasing temperature as the vibrational modes become active. -

Experimental Determination of Heat Capacities and Their Correlation with Theoretical Predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia
Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia Citation: Am. J. Phys. 79, 1099 (2011); doi: 10.1119/1.3625869 View online: http://dx.doi.org/10.1119/1.3625869 View Table of Contents: http://ajp.aapt.org/resource/1/AJPIAS/v79/i11 Published by the American Association of Physics Teachers Additional information on Am. J. Phys. Journal Homepage: http://ajp.aapt.org/ Journal Information: http://ajp.aapt.org/about/about_the_journal Top downloads: http://ajp.aapt.org/most_downloaded Information for Authors: http://ajp.dickinson.edu/Contributors/contGenInfo.html Downloaded 13 Aug 2013 to 157.92.4.6. Redistribution subject to AAPT license or copyright; see http://ajp.aapt.org/authors/copyright_permission Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar,a) and Wasif Zia School of Science and Engineering, Lahore University of Management Sciences (LUMS), Opposite Sector U, D. H. A., Lahore 54792, Pakistan (Received 25 January 2011; accepted 23 July 2011) We discuss an experiment for determining the heat capacities of various solids based on a calorimetric approach where the solid vaporizes a measurable mass of liquid nitrogen. We demonstrate our technique for copper and aluminum, and compare our data with Einstein’s model of independent harmonic oscillators and the more accurate Debye model. We also illustrate an interesting material property, the Verwey transition -

Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 Mpa
Volume 96, Number 6, November-December 1991 Journal of Research of the National Institute of Standards and Technology [J. Res. Nat!. Inst. Stand. Technol. 96, 725 (1991)] Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa Volume 96 Number 6 November-December 1991 J. W. Magee Molar heat capacities at constant vol- the Cv (fi,T) data and also show satis- ume (C,) for nitrogen have been mea- factory agreement with published heat National Institute of Standards sured with an automated adiabatic capacity data. The overall uncertainty and Technology, calorimeter. The temperatures ranged of the Cy values ranges from 2% in the Boulder, CO 80303 from 65 to 300 K, while pressures were vapor to 0.5% in the liquid. as high as 35 MPa. Calorimetric data were obtained for a total of 276 state Key words: calorimeter; heat capacity; conditions on 14 isochores. Extensive high pressure; isochorlc; liquid; mea- results which were obtained in the satu- surement; nitrogen; saturation; vapor. rated liquid region (C^^^' and C„) demonstrate the internal consistency of Accepted: August 30, 1991 Glossary ticular, the heat capacity at constant volume {Cv) is a,b,c,d,e Coefficients for Eq. (7) related to P(j^T) by: c. Molar heat capacity at constant volume a Molar heat capacity in the ideal gas state (1) C„<2> Molar heat capacity of a two-phase sample Jo a Molar heat capacity of a saturated liquid sample where C? is the ideal gas heat capacity. Conse- Vbamb Volume of the calorimeter containing sample quently, Cv measurements which cover a wide Vr, Molar volume, dm' • mol"' range of {P, p, T) states are beneficial to the devel- P Pressure, MPa opment of an accurate equation of state for the P. -

HEAT CAPACITY of MINERALS: a HANDS-ON INTRODUCTION to CHEMICAL THERMODYNAMICS David G
HEAT CAPACITY OF MINERALS: A HANDS-ON INTRODUCTION TO CHEMICAL THERMODYNAMICS David G. Bailey Hamilton College Clinton, NY 13323 email:[email protected] INTRODUCTION Minerals are inorganic chemical compounds with a wide range of physical and chemical properties. Geologists frequently measure and observe properties such as hardness, specific gravity, color, etc. Unfortunately, students usually view these properties simply as tools for identifying unknown mineral specimens. One of the objectives of this exercise is to make students aware of the fact that minerals have many additional properties that can be measured, and that all of the physical and chemical properties of minerals have important applications beyond that of simple mineral identification. ROCKS AS CHEMICAL SYSTEMS In order to understand fully many geological processes, the rocks and the minerals of which they are composed must be viewed as complex chemical systems. As with any natural system, minerals and rocks tend toward the lowest possible energy configuration. In order to assess whether or not certain minerals are stable, or if the various minerals in the rock are in equilibrium, one needs to know how much energy exists in the system, and how it is partitioned amongst the various phases. The energy in a system that is available for driving chemical reactions is referred to as the Gibbs Free Energy (GFE). For any chemical reaction, the reactants and the products are in equilibrium (i.e., they all are stable and exist in the system) if the GFE of the reactants is equal to that of the products. For example: reactants 12roducts CaC03 + Si02 CaSi03 + CO2 calcite + quartz wollastonite + carbon dioxide at equilibrium: (Gcalcite+ GqtJ = (Gwoll + GC02) or: ilG = (Greactants- Gproducts)= 0 If the free energy of the reactants and products are not equal, the reaction will proceed to the assemblage with the lowest total energy. -

A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 Mpa Roland Span, Eric W
A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 MPa Roland Span, Eric W. Lemmon, Richard T Jacobsen, Wolfgang Wagner, and Akimichi Yokozeki Citation: Journal of Physical and Chemical Reference Data 29, 1361 (2000); doi: 10.1063/1.1349047 View online: http://dx.doi.org/10.1063/1.1349047 View Table of Contents: http://scitation.aip.org/content/aip/journal/jpcrd/29/6?ver=pdfcov Published by the AIP Publishing Articles you may be interested in A Reference Equation of State for the Thermodynamic Properties of Ethane for Temperatures from the Melting Line to 675 K and Pressures up to 900 MPa J. Phys. Chem. Ref. Data 35, 205 (2006); 10.1063/1.1859286 A New Functional Form and New Fitting Techniques for Equations of State with Application to Pentafluoroethane (HFC-125) J. Phys. Chem. Ref. Data 34, 69 (2005); 10.1063/1.1797813 The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use J. Phys. Chem. Ref. Data 31, 387 (2002); 10.1063/1.1461829 New Equation of State for Ethylene Covering the Fluid Region for Temperatures From the Melting Line to 450 K at Pressures up to 300 MPa J. Phys. Chem. Ref. Data 29, 1053 (2000); 10.1063/1.1329318 Thermodynamic Properties of Air and Mixtures of Nitrogen, Argon, and Oxygen From 60 to 2000 K at Pressures to 2000 MPa J. Phys. Chem. Ref. Data 29, 331 (2000); 10.1063/1.1285884 This article is copyrighted as indicated in the article. -

Physical Chemistry I
Physical Chemistry I Andrew Rosen August 19, 2013 Contents 1 Thermodynamics 5 1.1 Thermodynamic Systems and Properties . .5 1.1.1 Systems vs. Surroundings . .5 1.1.2 Types of Walls . .5 1.1.3 Equilibrium . .5 1.1.4 Thermodynamic Properties . .5 1.2 Temperature . .6 1.3 The Mole . .6 1.4 Ideal Gases . .6 1.4.1 Boyle’s and Charles’ Laws . .6 1.4.2 Ideal Gas Equation . .7 1.5 Equations of State . .7 2 The First Law of Thermodynamics 7 2.1 Classical Mechanics . .7 2.2 P-V Work . .8 2.3 Heat and The First Law of Thermodynamics . .8 2.4 Enthalpy and Heat Capacity . .9 2.5 The Joule and Joule-Thomson Experiments . .9 2.6 The Perfect Gas . 10 2.7 How to Find Pressure-Volume Work . 10 2.7.1 Non-Ideal Gas (Van der Waals Gas) . 10 2.7.2 Ideal Gas . 10 2.7.3 Reversible Adiabatic Process in a Perfect Gas . 11 2.8 Summary of Calculating First Law Quantities . 11 2.8.1 Constant Pressure (Isobaric) Heating . 11 2.8.2 Constant Volume (Isochoric) Heating . 11 2.8.3 Reversible Isothermal Process in a Perfect Gas . 12 2.8.4 Reversible Adiabatic Process in a Perfect Gas . 12 2.8.5 Adiabatic Expansion of a Perfect Gas into a Vacuum . 12 2.8.6 Reversible Phase Change at Constant T and P ...................... 12 2.9 Molecular Modes of Energy Storage . 12 2.9.1 Degrees of Freedom . 12 1 2.9.2 Classical Mechanics . -

CHEM 477 Physical Chemistry Laboratory - 2
Colorado State University CHEM 477 Physical Chemistry Laboratory - 2 Notes for Heat-Capacity Ratio of Gases The following is a set of short notes to outline the experiment in question and to provide helpful guidance to those executing the experiment. A. The thermodynamic property of a gas consisting of the ratio of its heat capacity at constant pressure to its heat capacity at constant volume can be readily and accurately measured by the speed of acoustic waves, that is, sound in that gas. This measurement will be performed on several different gases. B. For an ideal gas the heat capacity ratio is understood by thermodynamic analysis of the configuration, that is, the number of motional degrees of freedom, of the gas. C. This is a straight-forward measurement but requires control over a number of experimental variables and a good understanding of related thermodynamics. D. Spend sufficient time learning how to use the apparatus in order to make high- quality measurements of the speed of sound using AIR only. E. Demonstrate to the teaching staff the quality of your measurements BEFORE attempting to use any other gas. F. Determine the relevance of the temperature and the pressure of the gas to the speed of sound within it. There is ample literature available to answer these two separate questions definitively, not trivially. G. Do not confuse heat capacity with heat capacity ratio. These are separate yet related. Your focus ought to be primarily and deeply on the heat capacity ratio. H. Review the reference by Garland et. al. and follow its Results and Discussion topics in your written laboratory report. -

Physical Chemistry-Ii
____________________________________________________________________________________________________ Subject CHEMISTRY Paper No and Title 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No and 13: Molar heat capacities and Electronic Partition Title function Module Tag CHE_P6_M13 Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Molar Heat Capacities – Solids 3.1 Einstein Theory of Heat Capacity of Solids 4. Partition function – Internal Structure of Atom 4.1 Electronic Partition Function 5. Summary Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to Learn about molar heat capacities of solids Learn about Einstein theory of heat capacities of solids Derive electronic partition function 2. Introduction In our previous module, we did talk about molar heat capacity of gases. Molar heat capacities provide a testing ground for verifying the theoretical