HEAT CAPACITY of MINERALS: a HANDS-ON INTRODUCTION to CHEMICAL THERMODYNAMICS David G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -
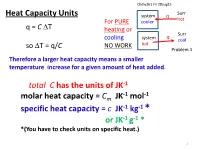
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

Experimental Determination of Heat Capacities and Their Correlation with Theoretical Predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia
Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia Citation: Am. J. Phys. 79, 1099 (2011); doi: 10.1119/1.3625869 View online: http://dx.doi.org/10.1119/1.3625869 View Table of Contents: http://ajp.aapt.org/resource/1/AJPIAS/v79/i11 Published by the American Association of Physics Teachers Additional information on Am. J. Phys. Journal Homepage: http://ajp.aapt.org/ Journal Information: http://ajp.aapt.org/about/about_the_journal Top downloads: http://ajp.aapt.org/most_downloaded Information for Authors: http://ajp.dickinson.edu/Contributors/contGenInfo.html Downloaded 13 Aug 2013 to 157.92.4.6. Redistribution subject to AAPT license or copyright; see http://ajp.aapt.org/authors/copyright_permission Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar,a) and Wasif Zia School of Science and Engineering, Lahore University of Management Sciences (LUMS), Opposite Sector U, D. H. A., Lahore 54792, Pakistan (Received 25 January 2011; accepted 23 July 2011) We discuss an experiment for determining the heat capacities of various solids based on a calorimetric approach where the solid vaporizes a measurable mass of liquid nitrogen. We demonstrate our technique for copper and aluminum, and compare our data with Einstein’s model of independent harmonic oscillators and the more accurate Debye model. We also illustrate an interesting material property, the Verwey transition -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 Mpa
Volume 96, Number 6, November-December 1991 Journal of Research of the National Institute of Standards and Technology [J. Res. Nat!. Inst. Stand. Technol. 96, 725 (1991)] Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa Volume 96 Number 6 November-December 1991 J. W. Magee Molar heat capacities at constant vol- the Cv (fi,T) data and also show satis- ume (C,) for nitrogen have been mea- factory agreement with published heat National Institute of Standards sured with an automated adiabatic capacity data. The overall uncertainty and Technology, calorimeter. The temperatures ranged of the Cy values ranges from 2% in the Boulder, CO 80303 from 65 to 300 K, while pressures were vapor to 0.5% in the liquid. as high as 35 MPa. Calorimetric data were obtained for a total of 276 state Key words: calorimeter; heat capacity; conditions on 14 isochores. Extensive high pressure; isochorlc; liquid; mea- results which were obtained in the satu- surement; nitrogen; saturation; vapor. rated liquid region (C^^^' and C„) demonstrate the internal consistency of Accepted: August 30, 1991 Glossary ticular, the heat capacity at constant volume {Cv) is a,b,c,d,e Coefficients for Eq. (7) related to P(j^T) by: c. Molar heat capacity at constant volume a Molar heat capacity in the ideal gas state (1) C„<2> Molar heat capacity of a two-phase sample Jo a Molar heat capacity of a saturated liquid sample where C? is the ideal gas heat capacity. Conse- Vbamb Volume of the calorimeter containing sample quently, Cv measurements which cover a wide Vr, Molar volume, dm' • mol"' range of {P, p, T) states are beneficial to the devel- P Pressure, MPa opment of an accurate equation of state for the P. -

Physical Chemistry-Ii
____________________________________________________________________________________________________ Subject CHEMISTRY Paper No and Title 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No and 13: Molar heat capacities and Electronic Partition Title function Module Tag CHE_P6_M13 Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Molar Heat Capacities – Solids 3.1 Einstein Theory of Heat Capacity of Solids 4. Partition function – Internal Structure of Atom 4.1 Electronic Partition Function 5. Summary Chemistry Paper No. 6: PHYSICAL CHEMISTRY-II (Statistical Thermodynamics, Chemical Dynamics, Electrochemistry and Macromolecules) Module No. 13: Molar heat capacities and Electronic Partition function ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to Learn about molar heat capacities of solids Learn about Einstein theory of heat capacities of solids Derive electronic partition function 2. Introduction In our previous module, we did talk about molar heat capacity of gases. Molar heat capacities provide a testing ground for verifying the theoretical -

Equipartition Theorem - Wikipedia 1 of 32
Equipartition theorem - Wikipedia 1 of 32 Equipartition theorem In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in translational motion of a molecule should equal that in rotational motion. The equipartition theorem makes quantitative Thermal motion of an α-helical predictions. Like the virial theorem, it gives the total peptide. The jittery motion is random average kinetic and potential energies for a system at a and complex, and the energy of any given temperature, from which the system's heat particular atom can fluctuate wildly. capacity can be computed. However, equipartition also Nevertheless, the equipartition theorem allows the average kinetic gives the average values of individual components of the energy of each atom to be energy, such as the kinetic energy of a particular particle computed, as well as the average or the potential energy of a single spring. For example, potential energies of many it predicts that every atom in a monatomic ideal gas has vibrational modes. The grey, red an average kinetic energy of (3/2)kBT in thermal and blue spheres represent atoms of carbon, oxygen and nitrogen, equilibrium, where kB is the Boltzmann constant and T respectively; the smaller white is the (thermodynamic) temperature. More generally, spheres represent atoms of equipartition can be applied to any classical system in hydrogen. -

Heats of Transition, Heats of Reaction, Specific Heats, And
Heats of Transition, Heats of Reaction, Specific Heats, and Hess's Law GOAL AND OVERVIEW A simple calorimeter will be made and calibrated. It will be used to determine the heat of fusion of ice, the specific heat of metals, and the heat of several chemical reactions. These heats of reaction will be used with Hess's law to determine another desired heat of reaction. Objectives and Science Skills • Understand, explain, and apply the concepts and uses of calorimetry. • Describe heat transfer processes quantitatively and qualitatively, including those related to heat capacity (including standard and molar), heat (enthalpy) of fusion, and heats (enthalpies) of chemical reactions. • Apply Hess's law using experimental data to determine an unknown heat (enthalpy) of reac- tion. • Quantitatively and qualitatively compare experimental results with theoretical values. • Identify and discuss factors or effects that may contribute to deviations between theoretical and experimental results and formulate optimization strategies. SUGGESTED REVIEW AND EXTERNAL READING • See reference section; textbook information on calorimetry and thermochemistry BACKGROUND The internal energy, E, of a system is not always a convenient property to work with when studying processes occurring at constant pressure. This would include many biological processes and chemical reactions done in lab. A more convenient property is the sum of the internal energy and pressure-volume work (−wsys = P ∆V at constant P). This is called the enthalpy of the system and is given the symbol H. H ≡ E + pV (definition of enthalpy; H) (1) Enthalpy is simply a corrected energy that reflects the change in energy of the system while it is allowed to expand or contract against a constant pressure. -

Entropy Change for the Irreversible Heat Transfer Between Two Finite
European Journal of Physics Eur. J. Phys. 36 (2015) 048004 (3pp) doi:10.1088/0143-0807/36/4/048004 Letters and Comments Entropy change for the irreversible heat transfer between two finite objects Carl E Mungan Physics Department, US Naval Academy, Annapolis, MD 21402-1363, USA E-mail: [email protected] Received 4 October 2014, revised 6 April 2015 Accepted for publication 15 May 2015 Published 10 June 2015 Abstract A positive entropy change is verified for an isolated system of two blocks of different initial temperatures and of different but finite heat capacities that are brought into contact with each other and allowed to fully thermalize. Keywords: thermalization, entropy, heat capacity, second law of thermodynamics A recent article by Lima considers the timescale on which an initially hot object thermalizes with an initially cold object when they are brought into contact with each other inside an insulated box [1]. For simplicity, both objects are assumed to have temperature-independent heat capacities. Another interesting aspect of this problem is to compute the entropy change during the process. Textbooks typically only cover the cases where either the objects have identical heat capacity, or one of the objects is a thermal reservoir (i.e., having infinite heat capacity) so that its temperature remains constant [2]. It is a useful exercise to algebraically verify the second law of thermodynamics for the more general case where the two objects have unequal heat capacities, both of which are finite. (From a calculus point of view, each time an increment of heat dQ is transferred from object A at variable temperature TA to object B at temperature TB, the entropy change of the Universe is dd/d/SQTQT=−AB + which is positive whenever TTAB> .) As sketched in figure 1 of Lima [1], the two blocks have initial absolute temperatures (in kelvin) of T1 and T2 where TT12>>0. -

7-Specific Heat of a Metal
Experiment 7 - Finding the Specific Heat of a Metal Heat is needed to warm up anything, but the amount of heat is not the same for all things. Therefore it is desirable to know the specific heat of a substance. The amount of heat needed to raise the temperature of one gram of a substance by one Celsius degree is call the “specific heat” or “specific heat capacity” of that substance. For pure water, that amount is one calorie, which is equivalent to 4.184 joules. Almost all other substances have lower specific heats than water does. Specific heat is defined in terms of one gram of substance and a one degree temperature rise, but of course it is applied to other-sized samples and other temperature changes. So, in general terms: Heat that must be put = (temperature change) × (grams sample) × (specific heat) into a sample or Q =s•m•∆T, where Q is the amount of heat, s is the specific heat, m is the mass of the sample, and ∆T is the temperature change. This equation can be used to calculate the amount of heat that must be involved when the other three values are known or measured. If a substance is cooling off, rather than warming up, the term Q represents the heat that is given off by the sample (instead of the heat that must be put into the sample). This same equation can also be used to calculate a specific heat, when the other three terms are known or measured. That is what you will be doing in the present experiment.