Heat Capacities: Q ∝ ∆T Q = C ∆T T ↑ ∆U T ↑ ∆H Q = C V ∆T Q = C V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -

21Ia'b;Q'c/Ablc (C/A)(C/B).__(C)(C/Ab)
ON THE q-ANALOG OF KUMMER’$ THEOREM AND APPLICATIONS GEORGE E. ANDREWS 1. Introduction. The q-analogs for Gauss’s summation of 2Fl[a, b; c; 1] and Saalschutz’s summation of 3F2[a, b, -n; c, a b c n 1; 1] are well known, namely, E. Heine [8; p. 107, Equation (6)] showed that (1.1) 21Ia’ b;q’c/ablc (c/a)(c/b).__(c)(c/ab) where - - and (a). (a; q), (1 -a)(1 -aq) (1 -aq-a), (a) (a; q),(R) lim,. (a),. (See also [12; p. 97, Equation (3.3.2.2)].) F. H. Jackson [9; p. 145] showed that b, q-"; q, (c/a),,(c/b). (c),,(c/ab), a2ia, c, abq/cq ql The q-analog of Dixon’s summation of 3F[a, b, c; 1 a b, 1 -[- a c; 1] was more difficult to find, and indeed only a partial analog is true; namely, W. N. Bailey [5] and F. tI. Jackson [10; p. 167, Equation (2)] proved that if a where n is a positive integer, then b, c; q, qa[ bc | (b/a),(c/a)(qa)(bca-). (1.3) 34 | ba-)-(ai (a- qa) bca-1), b,c There are three other well-known summations for the .F1 series, namely, Kummer’s theorem [12; p. 243, Equation (III. 5)] r(1%a-- b) (1.4) 2F[a, b; 1 a- b;--1] r(1 Gauss’s second theorem [12; p. 243, Equation III. 6)] Received December 23, 1972. The author ws partiMly supported by NtioaM Science Foundation Grat GP-23774. -
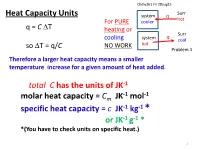
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Proposal for Generation Panel for Latin Script Label Generation Ruleset for the Root Zone
Generation Panel for Latin Script Label Generation Ruleset for the Root Zone Proposal for Generation Panel for Latin Script Label Generation Ruleset for the Root Zone Table of Contents 1. General Information 2 1.1 Use of Latin Script characters in domain names 3 1.2 Target Script for the Proposed Generation Panel 4 1.2.1 Diacritics 5 1.3 Countries with significant user communities using Latin script 6 2. Proposed Initial Composition of the Panel and Relationship with Past Work or Working Groups 7 3. Work Plan 13 3.1 Suggested Timeline with Significant Milestones 13 3.2 Sources for funding travel and logistics 16 3.3 Need for ICANN provided advisors 17 4. References 17 1 Generation Panel for Latin Script Label Generation Ruleset for the Root Zone 1. General Information The Latin script1 or Roman script is a major writing system of the world today, and the most widely used in terms of number of languages and number of speakers, with circa 70% of the world’s readers and writers making use of this script2 (Wikipedia). Historically, it is derived from the Greek alphabet, as is the Cyrillic script. The Greek alphabet is in turn derived from the Phoenician alphabet which dates to the mid-11th century BC and is itself based on older scripts. This explains why Latin, Cyrillic and Greek share some letters, which may become relevant to the ruleset in the form of cross-script variants. The Latin alphabet itself originated in Italy in the 7th Century BC. The original alphabet contained 21 upper case only letters: A, B, C, D, E, F, Z, H, I, K, L, M, N, O, P, Q, R, S, T, V and X. -

Defining Z in Q 75
Annals of Mathematics 183 (2016), 73{93 http://dx.doi.org/10.4007/annals.2016.183.1.2 Defining Z in Q By Jochen Koenigsmann Abstract We show that Z is definable in Q by a universal first-order formula in the language of rings. We also present an 89-formula for Z in Q with just one universal quantifier. We exhibit new diophantine subsets of Q like the complement of the image of the norm map under a quadratic extension, and we give an elementary proof for the fact that the set of nonsquares is diophantine. 1. Z is universally definable in Q Hilbert's 10th problem was to find a general algorithm for deciding, given n any n and any polynomial f 2 Z[x1; : : : ; xn], whether or not f has a zero in Z . Building on earlier work by Martin Davis, Hilary Putnam and Julia Robinson, Yuri Matiyasevich proved in 1970 that there can be no such algorithm. In particular, the existential first-order theory Th9(Z) of Z (in the language of rings Lring := f+; ·; 0; 1g) is undecidable. Hilbert's 10th problem over Q, i.e., the question whether Th9(Q) is decidable, is still open. If one had an existential (or diophantine) definition of Z in Q (i.e., a definition by an existential first-order Lring-formula), then Th9(Z) would be interpretable in Th9(Q), and the answer would, by Matiyasevich's Theorem, again be no. But it is still open whether Z is existentially definable in Q. The earliest first-order definition of Z in Q, which is due to Julia Robinson ([Rob49]), can be expressed by an 898-formula of the shape φ(t): 8x18x29y1 · · · 9y78z1 · · · 8z6 f(t; x1; x2; y1; : : : ; y7; z1; : : : ; z6) = 0 for some f 2 Z[t; x1; x2; y1; : : : ; y7; z1; : : : ; z6]; i.e., for any t 2 Q, t 2 Z if and only if φ(t) holds in Q: The research on this paper started while the author enjoyed the hospitality of the Max- Planck-Institut Bonn. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Special Characters in Aletheia
Special Characters in Aletheia Last Change: 28 May 2014 The following table comprises all special characters which are currently available through the virtual keyboard integrated in Aletheia. The virtual keyboard aids re-keying of historical documents containing characters that are mostly unsupported in other text editing tools (see Figure 1). Figure 1: Text input dialogue with virtual keyboard in Aletheia 1.2 Due to technical reasons, like font definition, Aletheia uses only precomposed characters. If required for other applications the mapping between a precomposed character and the corresponding decomposed character sequence is given in the table as well. When writing to files Aletheia encodes all characters in UTF-8 (variable-length multi-byte representation). Key: Glyph – the icon displayed in the virtual keyboard. Unicode – the actual Unicode character; can be copied and pasted into other applications. Please note that special characters might not be displayed properly if there is no corresponding font installed for the target application. Hex – the hexadecimal code point for the Unicode character Decimal – the decimal code point for the Unicode character Description – a short description of the special character Origin – where this character has been defined Base – the base character of the special character (if applicable), used for sorting Combining Character – combining character(s) to modify the base character (if applicable) Pre-composed Character Decomposed Character (used in Aletheia) (only for reference) Combining Glyph -

The Role of Gut Microbiota in an Ischemic Stroke
International Journal of Molecular Sciences Review The Role of Gut Microbiota in an Ischemic Stroke Ryszard Pluta 1,* , Sławomir Januszewski 1 and Stanisław J. Czuczwar 2 1 Laboratory of Ischemic and Neurodegenerative Brain Research, Mossakowski Medical Research Institute, Polish Academy of Sciences, 02-106 Warsaw, Poland; [email protected] 2 Department of Pathophysiology, Medical University of Lublin, 20-090 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-22-6086-540 Abstract: The intestinal microbiome, the largest reservoir of microorganisms in the human body, plays an important role in neurological development and aging as well as in brain disorders such as an ischemic stroke. Increasing knowledge about mediators and triggered pathways has contributed to a better understanding of the interaction between the gut-brain axis and the brain-gut axis. Intestinal bacteria produce neuroactive compounds and can modulate neuronal function, which affects behavior after an ischemic stroke. In addition, intestinal microorganisms affect host metabolism and immune status, which in turn affects the neuronal network in the ischemic brain. Here we discuss the latest results of animal and human research on two-way communication along the gut-brain axis in an ischemic stroke. Moreover, several reports have revealed the impact of an ischemic stroke on gut dysfunction and intestinal dysbiosis, highlighting the delicate play between the brain, intestines and microbiome after this acute brain injury. Despite our growing knowledge of intestinal microflora in shaping brain health, host metabolism, the immune system and disease progression, its therapeutic options in an ischemic stroke have not yet been fully utilized. -

Q: a Super-Quick, Articulation-Invariant Stroke-Gesture Recognizer for Low-Resource Devices
$Q: A Super-Quick, Articulation-Invariant Stroke-Gesture Recognizer for Low-Resource Devices Radu-Daniel Vatavu Lisa Anthony Jacob O. Wobbrock MintViz Lab MANSiD Center Department of CISE Information School DUB Group University Stefan cel Mare of Suceava University of Florida University of Washington Suceava 720229, Romania Gainesville, FL 32611, USA Seattle, WA 98195, USA [email protected] [email protected]fl.edu [email protected] ABSTRACT We introduce $Q, a super-quick, articulation-invariant point- cloud stroke-gesture recognizer for mobile, wearable, and embedded devices with low computing resources. $Q ran up to 142× faster than its predecessor $P in our benchmark eval- uations on several mobile CPUs, and executed in less than 3% of $P’s computations without any accuracy loss. In our most extreme evaluation demanding over 99% user-independent recognition accuracy, $P required 9.4s to run a single classifi- cation, while $Q completed in just 191ms (a 49× speed-up) on a Cortex-A7, one of the most widespread CPUs on the mobile market. $Q was even faster on a low-end 600-MHz processor, on which it executed in only 0.7% of $P’s computa- Figure 1. Classification times for $P and $Q (left) on five CPU architec- tures common for today’s mobile, wearable, and embedded platforms tions (a 142× speed-up), reducing classification time from two for a set of 16 distinct stroke-gestures with user-independent accuracy re- minutes to less than one second. $Q is the next major step for quirements of >99%. Speed-ups of 46× on average and up to 142× over the “$-family” of gesture recognizers: articulation-invariant, $P [50] make $Q very fast and suitable for next generation miniaturized extremely fast, accurate, and implementable on top of $P with low-resource devices, like the Gear Fit smartwatch shown at right. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

Experimental Determination of Heat Capacities and Their Correlation with Theoretical Predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia
Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia Citation: Am. J. Phys. 79, 1099 (2011); doi: 10.1119/1.3625869 View online: http://dx.doi.org/10.1119/1.3625869 View Table of Contents: http://ajp.aapt.org/resource/1/AJPIAS/v79/i11 Published by the American Association of Physics Teachers Additional information on Am. J. Phys. Journal Homepage: http://ajp.aapt.org/ Journal Information: http://ajp.aapt.org/about/about_the_journal Top downloads: http://ajp.aapt.org/most_downloaded Information for Authors: http://ajp.dickinson.edu/Contributors/contGenInfo.html Downloaded 13 Aug 2013 to 157.92.4.6. Redistribution subject to AAPT license or copyright; see http://ajp.aapt.org/authors/copyright_permission Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar,a) and Wasif Zia School of Science and Engineering, Lahore University of Management Sciences (LUMS), Opposite Sector U, D. H. A., Lahore 54792, Pakistan (Received 25 January 2011; accepted 23 July 2011) We discuss an experiment for determining the heat capacities of various solids based on a calorimetric approach where the solid vaporizes a measurable mass of liquid nitrogen. We demonstrate our technique for copper and aluminum, and compare our data with Einstein’s model of independent harmonic oscillators and the more accurate Debye model. We also illustrate an interesting material property, the Verwey transition -

Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 Mpa
Volume 96, Number 6, November-December 1991 Journal of Research of the National Institute of Standards and Technology [J. Res. Nat!. Inst. Stand. Technol. 96, 725 (1991)] Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa Volume 96 Number 6 November-December 1991 J. W. Magee Molar heat capacities at constant vol- the Cv (fi,T) data and also show satis- ume (C,) for nitrogen have been mea- factory agreement with published heat National Institute of Standards sured with an automated adiabatic capacity data. The overall uncertainty and Technology, calorimeter. The temperatures ranged of the Cy values ranges from 2% in the Boulder, CO 80303 from 65 to 300 K, while pressures were vapor to 0.5% in the liquid. as high as 35 MPa. Calorimetric data were obtained for a total of 276 state Key words: calorimeter; heat capacity; conditions on 14 isochores. Extensive high pressure; isochorlc; liquid; mea- results which were obtained in the satu- surement; nitrogen; saturation; vapor. rated liquid region (C^^^' and C„) demonstrate the internal consistency of Accepted: August 30, 1991 Glossary ticular, the heat capacity at constant volume {Cv) is a,b,c,d,e Coefficients for Eq. (7) related to P(j^T) by: c. Molar heat capacity at constant volume a Molar heat capacity in the ideal gas state (1) C„<2> Molar heat capacity of a two-phase sample Jo a Molar heat capacity of a saturated liquid sample where C? is the ideal gas heat capacity. Conse- Vbamb Volume of the calorimeter containing sample quently, Cv measurements which cover a wide Vr, Molar volume, dm' • mol"' range of {P, p, T) states are beneficial to the devel- P Pressure, MPa opment of an accurate equation of state for the P.