Thermodynamics, the First Law: the Concepts Thermodynamics – Concerned with the Studies of Transformation of Energy from Heat to Work and Vice Versa
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M
Purdue University Purdue e-Pubs CTRC Research Publications Cooling Technologies Research Center 2012 Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M. Flueckiger Purdue University Fabien Volle Laboratoire des Sciences des Procédés et des Matériaux S V. Garimella Purdue University, [email protected] Rajiv K. Mongia Intel Corporation Follow this and additional works at: http://docs.lib.purdue.edu/coolingpubs Flueckiger, Scott M.; Volle, Fabien; Garimella, S V.; and Mongia, Rajiv K., "Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump" (2012). CTRC Research Publications. Paper 182. http://dx.doi.org/http://dx.doi.org/10.1016/j.enconman.2012.04.015 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump* Scott M. Flueckiger1, Fabien Volle2, Suresh V. Garimella1**, Rajiv K. Mongia3 1 Cooling Technologies Research Center, an NSF I/UCRC School of Mechanical Engineering and Birck Nanotechnology Center 585 Purdue Mall, Purdue University West Lafayette, Indiana 47907-2088 USA 2 Laboratoire des Sciences des Procédés et des Matériaux (LSPM, UPR 3407 CNRS), Université Paris XIII, 99 avenue J. B. Clément, 93430 Villetaneuse, France 3 Intel Corporation Santa Clara, California 95054 USA * Submitted for publication in Energy Conversion and Management ** Author to who correspondence should be addressed: (765) 494-5621, [email protected] Abstract Representative reversible endothermic chemical reactions (paraldehyde depolymerization and 2-proponal dehydrogenation) are theoretically assessed for their use in a chemical heat pump design for compact thermal management applications. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

Chapter 3. Second and Third Law of Thermodynamics
Chapter 3. Second and third law of thermodynamics Important Concepts Review Entropy; Gibbs Free Energy • Entropy (S) – definitions Law of Corresponding States (ch 1 notes) • Entropy changes in reversible and Reduced pressure, temperatures, volumes irreversible processes • Entropy of mixing of ideal gases • 2nd law of thermodynamics • 3rd law of thermodynamics Math • Free energy Numerical integration by computer • Maxwell relations (Trapezoidal integration • Dependence of free energy on P, V, T https://en.wikipedia.org/wiki/Trapezoidal_rule) • Thermodynamic functions of mixtures Properties of partial differential equations • Partial molar quantities and chemical Rules for inequalities potential Major Concept Review • Adiabats vs. isotherms p1V1 p2V2 • Sign convention for work and heat w done on c=C /R vm system, q supplied to system : + p1V1 p2V2 =Cp/CV w done by system, q removed from system : c c V1T1 V2T2 - • Joule-Thomson expansion (DH=0); • State variables depend on final & initial state; not Joule-Thomson coefficient, inversion path. temperature • Reversible change occurs in series of equilibrium V states T TT V P p • Adiabatic q = 0; Isothermal DT = 0 H CP • Equations of state for enthalpy, H and internal • Formation reaction; enthalpies of energy, U reaction, Hess’s Law; other changes D rxn H iD f Hi i T D rxn H Drxn Href DrxnCpdT Tref • Calorimetry Spontaneous and Nonspontaneous Changes First Law: when one form of energy is converted to another, the total energy in universe is conserved. • Does not give any other restriction on a process • But many processes have a natural direction Examples • gas expands into a vacuum; not the reverse • can burn paper; can't unburn paper • heat never flows spontaneously from cold to hot These changes are called nonspontaneous changes. -

Work and Energy Summary Sheet Chapter 6
Work and Energy Summary Sheet Chapter 6 Work: work is done when a force is applied to a mass through a displacement or W=Fd. The force and the displacement must be parallel to one another in order for work to be done. F (N) W =(Fcosθ)d F If the force is not parallel to The area of a force vs. the displacement, then the displacement graph + W component of the force that represents the work θ d (m) is parallel must be found. done by the varying - W d force. Signs and Units for Work Work is a scalar but it can be positive or negative. Units of Work F d W = + (Ex: pitcher throwing ball) 1 N•m = 1 J (Joule) F d W = - (Ex. catcher catching ball) Note: N = kg m/s2 • Work – Energy Principle Hooke’s Law x The work done on an object is equal to its change F = kx in kinetic energy. F F is the applied force. 2 2 x W = ΔEk = ½ mvf – ½ mvi x is the change in length. k is the spring constant. F Energy Defined Units Energy is the ability to do work. Same as work: 1 N•m = 1 J (Joule) Kinetic Energy Potential Energy Potential energy is stored energy due to a system’s shape, position, or Kinetic energy is the energy of state. motion. If a mass has velocity, Gravitational PE Elastic (Spring) PE then it has KE 2 Mass with height Stretch/compress elastic material Ek = ½ mv 2 EG = mgh EE = ½ kx To measure the change in KE Change in E use: G Change in ES 2 2 2 2 ΔEk = ½ mvf – ½ mvi ΔEG = mghf – mghi ΔEE = ½ kxf – ½ kxi Conservation of Energy “The total energy is neither increased nor decreased in any process. -
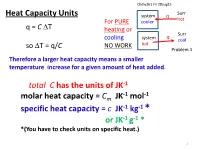
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Chemical Reactions Involve Energy Changes
Page 1 of 6 KEY CONCEPT Chemical reactions involve energy changes. BEFORE, you learned NOW, you will learn • Bonds are broken and made • About the energy in chemical during chemical reactions bonds between atoms • Mass is conserved in all • Why some chemical reactions chemical reactions release energy • Chemical reactions are • Why some chemical reactions represented by balanced absorb energy chemical equations VOCABULARY EXPLORE Energy Changes bond energy p. 86 How can you identify a transfer of energy? exothermic reaction p. 87 endothermic reaction p. 87 PROCEDURE MATERIALS photosynthesis p. 90 • graduated cylinder 1 Pour 50 ml of hot tap water into the cup • hot tap water and place the thermometer in the cup. • plastic cup 2 Wait 30 seconds, then record the • thermometer temperature of the water. • stopwatch • plastic spoon 3 Measure 5 tsp of Epsom salts. Add the Epsom salts to the beaker and immedi- • Epsom salts ately record the temperature while stirring the contents of the cup. 4 Continue to record the temperature every 30 seconds for 2 minutes. WHAT DO YOU THINK? • What happened to the temperature after you added the Epsom salts? • What do you think caused this change to occur? Chemical reactions release or absorb energy. COMBINATION NOTES Chemical reactions involve breaking bonds in reactants and forming Use combination notes new bonds in products. Breaking bonds requires energy, and forming to organize information on how chemical reactions bonds releases energy. The energy associated with bonds is called bond absorb or release energy. energy. What happens to this energy during a chemical reaction? Chemists have determined the bond energy for bonds between atoms. -

[email protected]
Fundamentals of mechanical engineering [email protected] MOHAMMED ABASS ALI What is thermodynamics Thermodynamics is the branch of physics that studies the effects of temperature and heat on physical systems at the macroscopic scale. In addition, it also studies the relationship that exists between heat, work and energy. Thermal energy is found in many forms in today’s society including power generation of electricity using gas, coal or nuclear, heating water by gas or electric. THE BASICS OF THERMODYNAMICS Basic concepts Properties are Features of a system which include mass, volume, energy, pressure and temperature. Thermodynamics also considers other quantities that are not physical properties, such as mass flow rates and energy transfers by work and heat. Energy forms Fluids and solids can possess several forms of energy. All fluids possess energy due to their temperature and this is referred to as ‘internal energy’. They will also possess ‘ potential energy’ (PE) due to distance (z) above a datum level and if the fluid is moving at a velocity (v), it will also possess ‘kinetic energy’. If the fluid is pressurised, it will possess ‘flow energy’ (FE). Pressure and temperature are the two governing factors and internal energy can be added to FE to produce a single property called ‘enthalpy’. Internal energy The molecules of a fluid possess both kinetic energy (KE) and PE relative to an internal datum. Generally, this is regarded simply as the energy due to its temperature and the change in internal energy in a fluid that undergoes a temperature change is given by ΔU = mcΔT The total internal energy is denoted by the symbol ‘U’, which has values of J, kJ or MJ; also the specific internal energy ‘u’ has the values of kJ/kg. -

Lecture 8: Maximum and Minimum Work, Thermodynamic Inequalities
Lecture 8: Maximum and Minimum Work, Thermodynamic Inequalities Chapter II. Thermodynamic Quantities A.G. Petukhov, PHYS 743 October 4, 2017 Chapter II. Thermodynamic Quantities Lecture 8: Maximum and Minimum Work, ThermodynamicA.G. Petukhov,October Inequalities PHYS 4, 743 2017 1 / 12 Maximum Work If a thermally isolated system is in non-equilibrium state it may do work on some external bodies while equilibrium is being established. The total work done depends on the way leading to the equilibrium. Therefore the final state will also be different. In any event, since system is thermally isolated the work done by the system: jAj = E0 − E(S); where E0 is the initial energy and E(S) is final (equilibrium) one. Le us consider the case when Vinit = Vfinal but can change during the process. @ jAj @E = − = −Tfinal < 0 @S @S V The entropy cannot decrease. Then it means that the greater is the change of the entropy the smaller is work done by the system The maximum work done by the system corresponds to the reversible process when ∆S = Sfinal − Sinitial = 0 Chapter II. Thermodynamic Quantities Lecture 8: Maximum and Minimum Work, ThermodynamicA.G. Petukhov,October Inequalities PHYS 4, 743 2017 2 / 12 Clausius Theorem dS 0 R > dS < 0 R S S TA > TA T > T B B A δQA > 0 B Q 0 δ B < The system following a closed path A: System receives heat from a hot reservoir. Temperature of the thermostat is slightly larger then the system temperature B: System dumps heat to a cold reservoir. Temperature of the system is slightly larger then that of the thermostat Chapter II. -

Internal Energy in an Electric Field
Internal energy in an electric field In an electric field, if the dipole moment is changed, the change of the energy is, U E P Using Einstein notation dU Ek dP k This is part of the total derivative of U dU TdSij d ij E kK dP H l dM l Make a Legendre transformation to the Gibbs potential G(T, H, E, ) GUTSijij EP kK HM l l SGTE data for pure elements http://www.sciencedirect.com/science/article/pii/036459169190030N Gibbs free energy GUTSijij EP kK HM l l dG dU TdS SdTijij d ij d ij E kk dP P kk dE H l dM l M l dH l dU TdSij d ij E kK dP H l dM l dG SdTij d ij P k dE k M l dH l G G G G total derivative: dG dT d dE dH ij k l T ij EH k l G G ij Pk E ij k G G M l S Hl T Direct and reciprocal effects (Maxwell relations) Useful to check for errors in experiments or calculations Maxwell relations Useful to check for errors in experiments or calculations Point Groups Crystals can have symmetries: rotation, reflection, inversion,... x 1 0 0 x y 0 cos sin y z 0 sin cos z Symmetries can be represented by matrices. All such matrices that bring the crystal into itself form the group of the crystal. AB G for A, B G 32 point groups (one point remains fixed during transformation) 230 space groups Cyclic groups C2 C4 http://en.wikipedia.org/wiki/Cyclic_group 2G Pyroelectricity i Ei T Pyroelectricity is described by a rank 1 tensor Pi i T 1 0 0 x x x 0 1 0 y y y 0 0 1 z z 0 1 0 0 x x 0 0 1 0 y y 0 0 0 1 z z 0 Pyroelectricity example Turmalin: point group 3m Quartz, ZnO, LaTaO 3 for T = 1°C, E ~ 7 ·104 V/m Pyroelectrics have a spontaneous polarization. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Estimation of Internal Pressure of Liquids and Liquid Mixtures
Available online at www.ilcpa.pl International Letters of Chemistry, Physics and Astronomy 5 (2012) 1-7 ISSN 2299-3843 Estimation of internal pressure of liquids and liquid mixtures N. Santhi 1, P. Sabarathinam 2, G. Alamelumangai 1, J. Madhumitha 1, M. Emayavaramban 1 1Department of Chemistry, Government Arts College, C.Mutlur, Chidambaram-608102, India 2Retd. Registrar & Head of the Department of Technology, Annamalai University, Annamalainagar-608002, India E-mail address: [email protected] ABSTRACT By combining the van der Waals’ equation of state and the Free Length Theory of Jacobson, a new theoretical model is developed for the prediction of internal pressure of pure liquids and liquid mixtures. It requires only the molar volume data in addition to the ratio of heat capacities and critical temperature. The proposed model is simple, reliably accurate and capable of predicting internal pressure of pure liquids with an average absolute deviation of 4.24% in the predicted internal pressure values compared to those given in literature. The average absolute deviation in the predicted internal pressure values through the proposed model for the five binary liquid mixtures tested varies from 0.29% to 1.9% when compared to those of literature values. Keywords: van der Waals, pressure of liquids, theory of Jacobson, capacity of heat liquids, equation of Srivastava and Berkowitz 1. INTRODUTION The importance of internal pressure in understanding the properties of liquids and the full potential of internal pressure as a structural probe did become apparent with the pioneering work of Hildebrand 1, 2 and the first review of the subject by Richards 3 appeared in 1925. -

Fundamentals of Hypersonic Flow - Aerothermodynamics
FUNDAMENTALSOFHYPERSONICFLOW- AEROTHERMODYNAMICS D. G. Fletcher von Karman Institute, Belgium 1. Introduction To aid in understanding the different topics discussed in the current Lecture Series and to provide background material for interested participants who may not have specialized training in this field, a brief summary of the fundamental attributes of hypersonic flows is given. Many of the topics that are introduced in this section will be elaborated further in contributions related to specific subjects related to sustained hypersonic flight. The differencesbetweenthethermalandchemicalaspectsofhypersonicflowandsupersonic flow are therefore highlighted. The age of some of the figures used in the subsequent discussion reflect the fact that the problems of hypersonic flight are not newly discovered! Fig. 1.1 Flight trajectories for different hypersonic vehicles comparing sustained atmo- spheric flight with re-entry. The hypersonic flight regime includes atmospheric entry and re-entry, ground testing, and flight for both powered and unpowered vehicles. In the present Lecture Series, the main interest is on sustained and controlled hypersonic flight, whether for military or civil transport application. Even though it is not currently certified for flight, there is one operational hypersonic vehicle: the space shuttle of NASA. At least 20 years before the development of the shuttle a significant activity in hypersonic flight research was conducted by the US Air Force in their X-15 program. This vehicle has reached a flight Mach number of 6.7 on its final flight, which also used to test a hypersonic ramjet engine. Direct shock impingement on the pylon holding a dummy engine caused severe heating and structural damage, and this was one of many lessons learned from the program.