Heat Capacity 1 Heat Capacity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Temperature Dependence of Breakdown and Avalanche Multiplication in In0.53Ga0.47As Diodes and Heterojunction Bipolar Transistors
This is a repository copy of Temperature dependence of breakdown and avalanche multiplication in In0.53Ga0.47As diodes and heterojunction bipolar transistors . White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/896/ Article: Yee, M., Ng, W.K., David, J.P.R. et al. (3 more authors) (2003) Temperature dependence of breakdown and avalanche multiplication in In0.53Ga0.47As diodes and heterojunction bipolar transistors. IEEE Transactions on Electron Devices, 50 (10). pp. 2021-2026. ISSN 0018-9383 https://doi.org/10.1109/TED.2003.816553 Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ IEEE TRANSACTIONS ON ELECTRON DEVICES, VOL. 50, NO. 10, OCTOBER 2003 2021 Temperature Dependence of Breakdown and Avalanche Multiplication in InHXSQGaHXRUAs Diodes and Heterojunction Bipolar Transistors M. -

Lab: Specific Heat
Lab: Specific Heat Summary Students relate thermal energy to heat capacity by comparing the heat capacities of different materials and graphing the change in temperature over time for a specific material. Students explore the idea of insulators and conductors in an attempt to apply their knowledge in real world applications, such as those that engineers would. Engineering Connection Engineers must understand the heat capacity of insulators and conductors if they wish to stay competitive in “green” enterprises. Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. In areas of cooler climates engineers chose building materials with a low heat capacity such as slate roofs in an attempt to heat the home during the day. In areas of warmer climates tile roofs are popular for their ability to store the sun's heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances. Just think about your aluminum soda can versus glass bottles! Grade Level: 9-12 Group Size: 4 Time Required: 90 minutes Activity Dependency :None Expendable Cost Per Group : US$ 5 The cost is less if thermometers are available for each group. NYS Science Standards: STANDARD 1 Analysis, Inquiry, and Design STANDARD 4 Performance indicator 2.2 Keywords: conductor, energy, energy storage, heat, specific heat capacity, insulator, thermal energy, thermometer, thermocouple Learning Objectives After this activity, students should be able to: Define heat capacity. Explain how heat capacity determines a material's ability to store thermal energy. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -

PHYS 596 Team 1
Braun, S., Ronzheimer, J., Schreiber, M., Hodgman, S., Rom, T., Bloch, I. and Schneider, U. (2013). Negative Absolute Temperature for Motional Degrees of Freedom. Science, 339(6115), pp.52-55. PHYS 596 Team 1 Shreya, Nina, Nathan, Faisal What is negative absolute temperature? • An ensemble of particles is said to have negative absolute temperature if higher energy states are more likely to be occupied than lower energy states. −퐸푖/푘푇 푃푖 ∝ 푒 • If high energy states are more populated than low energy states, entropy decreases with energy. 1 휕푆 ≡ 푇 휕퐸 Negative absolute temperature Previous work on negative temperature • The first experiment to measure negative temperature was performed at Harvard by Purcell and Pound in 1951. • By quickly reversing the magnetic field acting on a nuclear spin crystal, they produced a sample where the higher energy states were more occupied. • Since then negative temperature ensembles in spin systems have been produced in other ways. Oja and Lounasmaa (1997) gives a comprehensive review. Negative temperature for motional degrees of freedom • For the probability distribution of a negative temperature ensemble to be normalizable, we need an upper bound in energy. • Since localized spin systems have a finite number of energy states there is a natural upper bound in energy. • This is hard to achieve in systems with motional degrees of freedom since kinetic energy is usually not bounded from above. • Braun et al (2013) achieves exactly this with bosonic cold atoms in an optical lattice. What is the point? • At thermal equilibrium, negative temperature implies negative pressure. • This is relevant to models of dark energy and cosmology based on Bose-Einstein condensation. -

Compression of an Ideal Gas with Temperature Dependent Specific
Session 3666 Compression of an Ideal Gas with Temperature-Dependent Specific Heat Capacities Donald W. Mueller, Jr., Hosni I. Abu-Mulaweh Engineering Department Indiana University–Purdue University Fort Wayne Fort Wayne, IN 46805-1499, USA Overview The compression of gas in a steady-state, steady-flow (SSSF) compressor is an important topic that is addressed in virtually all engineering thermodynamics courses. A typical situation encountered is when the gas inlet conditions, temperature and pressure, are known and the compressor dis- charge pressure is specified. The traditional instructional approach is to make certain assumptions about the compression process and about the gas itself. For example, a special case often consid- ered is that the compression process is reversible and adiabatic, i.e. isentropic. The gas is usually ÊÌ considered to be ideal, i.e. ÈÚ applies, with either constant or temperature-dependent spe- cific heat capacities. The constant specific heat capacity assumption allows for direct computation of the discharge temperature, while the temperature-dependent specific heat assumption does not. In this paper, a MATLAB computer model of the compression process is presented. The substance being compressed is air which is considered to be an ideal gas with temperature-dependent specific heat capacities. Two approaches are presented to describe the temperature-dependent properties of air. The first approach is to use the ideal gas table data from Ref. [1] with a look-up interpolation scheme. The second approach is to use a curve fit with the NASA Lewis coefficients [2,3]. The computer model developed makes use of a bracketing–bisection algorithm [4] to determine the temperature of the air after compression. -

On the Equation of State of an Ideal Monatomic Gas According to the Quantum-Theory, In: KNAW, Proceedings, 16 I, 1913, Amsterdam, 1913, Pp
Huygens Institute - Royal Netherlands Academy of Arts and Sciences (KNAW) Citation: W.H. Keesom, On the equation of state of an ideal monatomic gas according to the quantum-theory, in: KNAW, Proceedings, 16 I, 1913, Amsterdam, 1913, pp. 227-236 This PDF was made on 24 September 2010, from the 'Digital Library' of the Dutch History of Science Web Center (www.dwc.knaw.nl) > 'Digital Library > Proceedings of the Royal Netherlands Academy of Arts and Sciences (KNAW), http://www.digitallibrary.nl' - 1 - 227 high. We are uncertain as to the cause of this differencc: most probably it is due to an nncertainty in the temperature with the absolute manometer. It is of special interest to compare these observations with NERNST'S formula. Tbe fourth column of Table II contains the pressUl'es aceord ing to this formula, calc111ated witb the eonstants whieb FALCK 1) ha~ determined with the data at bis disposal. FALOK found the following expression 6000 1 0,009983 log P • - --. - + 1. 7 5 log T - T + 3,1700 4,571 T . 4,571 where p is the pressure in atmospheres. The correspondence will be se en to be satisfactory considering the degree of accuracy of the observations. 1t does riot look as if the constants could be materially improved. Physics. - "On the equation of state oj an ideal monatomic gflS accoJ'ding to tlw quantum-the01'Y." By Dr. W. H. KEEsmr. Supple ment N°. 30a to the Oommunications ft'om the PhysicaL Labora tory at Leiden. Oommunicated by Prof. H. KAl\IERLINGH ONNES. (Communicated in the meeting of May' 31, 1913). -

Consistent Thermostatistics Forbids Negative Absolute Temperatures
ARTICLES PUBLISHED ONLINE: 8 DECEMBER 2013 | DOI: 10.1038/NPHYS2815 Consistent thermostatistics forbids negative absolute temperatures Jörn Dunkel1* and Stefan Hilbert2 Over the past 60 years, a considerable number of theories and experiments have claimed the existence of negative absolute temperature in spin systems and ultracold quantum gases. This has led to speculation that ultracold gases may be dark-energy analogues and also suggests the feasibility of heat engines with efficiencies larger than one. Here, we prove that all previous negative temperature claims and their implications are invalid as they arise from the use of an entropy definition that is inconsistent both mathematically and thermodynamically. We show that the underlying conceptual deficiencies can be overcome if one adopts a microcanonical entropy functional originally derived by Gibbs. The resulting thermodynamic framework is self-consistent and implies that absolute temperature remains positive even for systems with a bounded spectrum. In addition, we propose a minimal quantum thermometer that can be implemented with available experimental techniques. ositivity of absolute temperature T, a key postulate of Gibbs formalism yields strictly non-negative absolute temperatures thermodynamics1, has repeatedly been challenged both even for quantum systems with a bounded spectrum, thereby Ptheoretically2–4 and experimentally5–7. If indeed realizable, invalidating all previous negative temperature claims. negative temperature systems promise profound practical and conceptual -
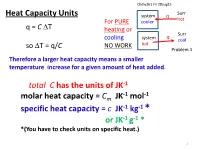
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures Over a Wide Temperature Range
entropy Article Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures over a Wide Temperature Range Sebastian Haas 1, Mike Mosbacher 1, Oleg N. Senkov 2 ID , Michael Feuerbacher 3, Jens Freudenberger 4,5 ID , Senol Gezgin 6, Rainer Völkl 1 and Uwe Glatzel 1,* ID 1 Metals and Alloys, University Bayreuth, 95447 Bayreuth, Germany; [email protected] (S.H.); [email protected] (M.M.); [email protected] (R.V.) 2 UES, Inc., 4401 Dayton-Xenia Rd., Dayton, OH 45432, USA; [email protected] 3 Institut für Mikrostrukturforschung, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] 4 Leibniz Institute for Solid State and Materials Research Dresden (IFW Dresden), 01069 Dresden, Germany; [email protected] 5 TU Bergakademie Freiberg, Institut für Werkstoffwissenschaft, 09599 Freiberg, Germany 6 NETZSCH Group, Analyzing & Testing, 95100 Selb, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-921-55-5555 Received: 31 July 2018; Accepted: 20 August 2018; Published: 30 August 2018 Abstract: We determined the entropy of high entropy alloys by investigating single-crystalline nickel and five high entropy alloys: two fcc-alloys, two bcc-alloys and one hcp-alloy. Since the configurational entropy of these single-phase alloys differs from alloys using a base element, it is important to quantify ◦ the entropy. Using differential scanning calorimetry, cp-measurements are carried out from −170 C to the materials’ solidus temperatures TS. From these experiments, we determined the thermal entropy and compared it to the configurational entropy for each of the studied alloys. -

Ideal Gasses Is Known As the Ideal Gas Law
ESCI 341 – Atmospheric Thermodynamics Lesson 4 –Ideal Gases References: An Introduction to Atmospheric Thermodynamics, Tsonis Introduction to Theoretical Meteorology, Hess Physical Chemistry (4th edition), Levine Thermodynamics and an Introduction to Thermostatistics, Callen IDEAL GASES An ideal gas is a gas with the following properties: There are no intermolecular forces, except during collisions. All collisions are elastic. The individual gas molecules have no volume (they behave like point masses). The equation of state for ideal gasses is known as the ideal gas law. The ideal gas law was discovered empirically, but can also be derived theoretically. The form we are most familiar with, pV nRT . Ideal Gas Law (1) R has a value of 8.3145 J-mol1-K1, and n is the number of moles (not molecules). A true ideal gas would be monatomic, meaning each molecule is comprised of a single atom. Real gasses in the atmosphere, such as O2 and N2, are diatomic, and some gasses such as CO2 and O3 are triatomic. Real atmospheric gasses have rotational and vibrational kinetic energy, in addition to translational kinetic energy. Even though the gasses that make up the atmosphere aren’t monatomic, they still closely obey the ideal gas law at the pressures and temperatures encountered in the atmosphere, so we can still use the ideal gas law. FORM OF IDEAL GAS LAW MOST USED BY METEOROLOGISTS In meteorology we use a modified form of the ideal gas law. We first divide (1) by volume to get n p RT . V we then multiply the RHS top and bottom by the molecular weight of the gas, M, to get Mn R p T . -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom.