Physics, Chapter 44: Stable Nuclei
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Nuclear Energy Agency Nuclear Data Committee
NUCLEAR ENERGY AGENCY NUCLEAR DATA COMMITTEE SUMMARY RECORD OF THE lWEEFPY-FIRST MEETING (Technical Sessions) CBNM, Gee1 (Belgium) 24th-28th September 1979 Compiled by C. COCEVA (Scientific Secretary) OECD NUCLEAR ENERGY AGENCY 38 Bd. Suchet, 75016 Paris TABLE OF CONTENTS TECHNICAL SESSIONS Participants in meeting 1. Isotopes 2. National Progress Reports 3. Meetings 4. Technical Discussions 5. Topical Meeting on "Progress in Neutron Data of Structural Materials for Fast ~eactors" 6. Neutron and Related Nuclear Data Compilations and Evaluations Appendices 1 Meetings of the IAEA/NDS planned for 1980, 1981 and 1982 2 Progranme of the Topical Meeting on "Progress in Neutron Data of Structural Materials for Fast Reactors " 3 Summary of the general discussion on the works presented at the Topical Meeting TECmTICAL SESSIONS Perticipants in the 21st Meeting were as follows : For Canada : Dr. W.G. Cross Atomic Energy of Canada Ltd. Chalk River For Japan : Dr. K. Tsukada Japan Atomic Energy Research Institute Tokai-blur a For the United States of America : Dr. R.E. Chrien (Chairman) Brookhaven National Laboratory Dr. S.L. Wl~etstone U.S. Department of Energy Dr. 8.T. Motz Los Alamos Scientific Laboratory Dr. F.G. Perey Oak Ridge National Laboratory For the countries of the European Communities and the European Commission acting together : Dr. R. Iiockhoff (Local Secretary) Central Bureau for Nuclear Pleasurements Geel, Belgium Dr. C. Coceva (Scientific Secretary) Comitato Nazionale per 1'Energia Nucleare Bologna, Italy Dr. S. Cierjacks Kernforschungszentrum Karlsruhe Federal Republic of Germany Dr. C. Fort Conunissariat i 1'Energie Atomique Cadaroche, France Dr. A. Michaudon (Vice-chairman) Commissariat 2 1'Energie Atomique Bruysrcs-1.e-ChZtel Dr. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology
Physical Science International Journal 15(2): 1-25, 2017; Article no.PSIJ.34889 ISSN: 2348-0130 Metastable Non-Nucleonic States of Nuclear Matter: Phenomenology Timashev Serge 1,2* 1Karpov Institute of Physical Chemistry, Moscow, Russia. 2National Research Nuclear University MEPhI, Moscow, Russia. Author’s contribution The sole author designed, analyzed and interpreted and prepared the manuscript. Article Information DOI: 10.9734/PSIJ/2017/34889 Editor(s): (1) Prof. Yang-Hui He, Professor of Mathematics, City University London, UK And Chang-Jiang Chair Professor in Physics and Qian-Ren Scholar, Nan Kai University, China & Tutor and Quondam-Socius in Mathematics, Merton College, University of Oxford, UK. (2) Roberto Oscar Aquilano, School of Exact Science, National University of Rosario (UNR),Rosario, Physics Institute (IFIR)(CONICET-UNR), Argentina. Reviewers: (1) Alejandro Gutiérrez-Rodríguez, Universidad Autónoma de Zacatecas, Mexico. (2) Arun Goyal, Delhi University, India. (3) Stanislav Fisenko, Moscow State Linguistic University, Russia. Complete Peer review History: http://www.sciencedomain.org/review-history/20031 Received 17 th June 2017 Accepted 8th July 2017 Original Research Article th Published 13 July 2017 ABSTRACT A hypothesis of the existence of metastable states for nuclear matter with a locally shaken-up nucleonic structure of the nucleus, was proposed earlier. Such states are initiated by inelastic scattering of electrons by nuclei along the path of weak nuclear interaction. The relaxation of such nuclei is also determined by weak interactions. The use of the hypothesis makes it possible to physically interpret a rather large group of experimental data on the initiation of low energy nuclear reactions (LENRs) and the acceleration of radioactive α- and β-decays in a low-temperature plasma. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -
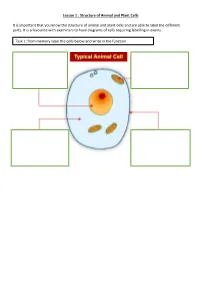
Lesson 1 : Structure of Animal and Plant Cells
Lesson 1 : Structure of Animal and Plant Cells It is important that you know the structure of animal and plant cells and are able to label the different parts. It is a favourite with examiners to have diagrams of cells requiring labelling in exams. Task 1: from memory label the cells below and write in the function Check your answers: There are many similarities and differences between animal and plant cells. Make sure you know these. Similarities Differences 1. Have a nucleus 1. Plant cells have a cellulose cell wall 2. Have a cytoplasm 2. Plant cells have a vacuole containing cell sap 3. Have a cell 3. Plant cells have chloroplast membrane 4. Contain 4. Many plant cells have a box-like shape whilst animal cell shape varies mitochondria 5. Plant cells have the nucleus to the side of the cell, animal cells have a nucleus in 5. Contain ribosomes the middle Task 2: Complete the sentences by filling in the gaps. Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. Check your answers: Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. -

Physics of Superheavy Elements Kouichi Hagino
Frontiers in Science II 2013.11.6 Physics of superheavy elements Kouichi Hagino Nuclear Theory Group, Department of Physics, Tohoku University What is nuclear physics? What are superheavy elements? How to create superheavy elements? What are chemical properties of superheavy elements? Introduction: atoms and atomic nuclei What would you see if you magnified the dog? ~ 50 cm Introduction: atoms and atomic nuclei cells ~ 50 cm ~ m = 10-6 m Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ m = 10-6 m ~ 10 m atom All things are made of atoms. ~ 10-10 m All things are made of atoms. • Thales, Democritus (ancient Greek) • Dalton (chemist, 19th century) • Boltzmann(19th century) • Einstein (1905) ~ 10-10 m STM image (surface physics group, Tohoku university) Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ 10 m atom atomic nucleus ~ 10-15 m ~ 10-10 m proton (+e) neutron (no charge) electron cloud (-e) Neutral atoms: # of protons = # of electrons Chemical properties of atoms # of electrons Mp ~ Mn ~ 2000 Me the mass of atom ~ the mass of nucleus Periodic table of chemical elements tabular arrangement of chemical elements based on the atomic numbers (= # of electrons = # of protons) What are we made of ? oxygen 43 kg cerium 40 mg gallium 0.7 mg carbon 16 kg barium 22 mg tellurium 0.7 mg hydrogen 7 kg iodine 20 mg yttrium 0.6 mg nitrogen 1.8 kg tin 20 mg bismuth 0.5 mg calcium 1.0 kg titanium 20 mg thallium 0.5 mg phosphorus 780 g boron 18 mg indium 0.4 mg potassium 140 g nickel 15 mg gold 0.2 mg sulphur 140 g selenium -

Section 29-4: the Chart of the Nuclides
29-4 The Chart of the Nuclides A nuclide is an atom that is characterized by what is in its nucleus. In other words, it is characterized by the number of protons it has, and by the number of neutrons it has. Figure 29.2 shows the chart of the nuclides, which plots, for stable and radioactive nuclides, the value of Z (the atomic number), on the vertical axis, and the value of N (the number of neutrons) on the horizontal axis. If you look at a horizontal line through the chart, you will find nuclides that all have the same number of protons, but a different number of neutrons. These are all nuclides of the same element, and are known as isotopes (equal proton number, different neutron number). On a vertical line through the chart, the nuclides all have the same number of neutrons, but a different number of protons. These nuclides are known as isotones (equal neutron number, different proton number). It is interesting to think about how the various decay processes play a role in the chart of the nuclides. Only the nuclides shown in black are stable. The stable nuclides give the chart a line of stability that goes from the bottom left toward the upper right, curving down and away from the Z = N line as you move toward higher N values. There are many more nuclides that are shown on the chart but which are not stable - these nuclides decay by means of a radioactive decay process. In general, the dominant decay process for a particular nuclide is the process that produces a nucleus closer to the line of stability than where it started. -

CROSS SECTIONS of (N,P), (N,Α), (N,2N) REACTIONS on ISOTOPES
CROSS SECTIONS OF (n, p), (n, α), (n, 2n) REACTIONS ON ISOTOPES OF Dy, Er, Yb AT En= 14.6 MEV А.O. Kadenko, O.M. Gorbachenko, V.A. Plujko, G.I. Primenko Nuclear Physics Department, Taras Shevchenko National University, Kyiv, Ukraine Abstract The cross sections of the neutron reactions at En= 14.6 MeV on the isotopes of Dy, Er, Yb with emission of neutrons, proton and alpha-particle were studied by the use of new experimental data and different theoretical approaches. New and improved experimental data were obtained by the neutron- activation technique. Present experimental results and evaluated nuclear data from EXFOR, TENDL, ENDF data libraries were compared with different systematics and calculations within codes of EMPIRE 3.0 and TALYS 1.2. Contribution of pre-equilibrium decay was studied. The recommendations on validity of different systematics for estimations of cross-sections of considered reactions are given. 1. Introduction Studies of the nuclear reaction cross sections induced by neutrons provides an opportunity to get information on the excited states of atomic nuclei and nuclear reaction mechanisms [1]. In addition, nuclear reaction cross section data are necessary in applied applications such as the design of fusion reactors protection and modernization of existing nuclear power plants [2, 3]. In particular, they allow to calculate the activity and the degree of radiation damage to structural elements of nuclear reactors [3]. Practical interest in cross sections of nuclear reactions caused by their wide use in experimental nuclear physics methods, such as the method of boundary indicators in measuring the spectrum of neutrons in the reactor core of a nuclear reactor, or neutron activation analysis. -

Module01 Nuclear Physics and Reactor Theory
Module I Nuclear physics and reactor theory International Atomic Energy Agency, May 2015 v1.0 Background In 1991, the General Conference (GC) in its resolution RES/552 requested the Director General to prepare 'a comprehensive proposal for education and training in both radiation protection and in nuclear safety' for consideration by the following GC in 1992. In 1992, the proposal was made by the Secretariat and after considering this proposal the General Conference requested the Director General to prepare a report on a possible programme of activities on education and training in radiological protection and nuclear safety in its resolution RES1584. In response to this request and as a first step, the Secretariat prepared a Standard Syllabus for the Post- graduate Educational Course in Radiation Protection. Subsequently, planning of specialised training courses and workshops in different areas of Standard Syllabus were also made. A similar approach was taken to develop basic professional training in nuclear safety. In January 1997, Programme Performance Assessment System (PPAS) recommended the preparation of a standard syllabus for nuclear safety based on Agency Safely Standard Series Documents and any other internationally accepted practices. A draft Standard Syllabus for Basic Professional Training Course in Nuclear Safety (BPTC) was prepared by a group of consultants in November 1997 and the syllabus was finalised in July 1998 in the second consultants meeting. The Basic Professional Training Course on Nuclear Safety was offered for the first time at the end of 1999, in English, in Saclay, France, in cooperation with Institut National des Sciences et Techniques Nucleaires/Commissariat a l'Energie Atomique (INSTN/CEA). -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Isotonic and Isotopic Dependence of the Radiative Neutron Capture Cross-Section on the Neutron Excess
ISOTONIC AND ISOTOPIC DEPENDENCE OF THE RADIATIVE NEUTRON CAPTURE CROSS-SECTION ON THE NEUTRON EXCESS Yu. N. Trofimov V.G. Khlopin Radium Institute, Leningrad Abstract The radiative neutron capture cross-section of nuclei has been derived as a function of neutron excess on the basis of the exponential dependence of the cross-section on the reaction energy. It is shown that unknown cross-sections of stable and radioactive nuclei may be evaluated by using the isotonic and isotopic dependence together with available reference cross-section measurements. Data on fast neutron radiative capture (P3) cross-sections for medium and heavy mass nuclei are at the present time fragmentary and in some cases highly contradictory. This is particularly true of naU [1] where the error of the evaluated cross-section is 50%. The present study is a sequel to the work initiated earlier [2] on the systematic dependence of the P3 neutron cross-section on the isotope mass number, an,r(A). On the basis of the systematics of the P3 neutron cross-sections it is possible: - to reject contradictory or questionable data, and - to predict cross-section values of nuclei which are difficult to measure, including those nuclei which are radioactive. On the basis of the evaluation of P3 neutron cross-sections for nuclei of given parity (three parity groups are taken into consideration here: N and Z even-even, even-odd and odd-even nuclei), the isotopic and isotonic dependence of the cross- section can be represented by o. =K1exp(-K2 a) (1) where a - is the P3 neutron cross-section, or-(N-Z)/A - is the relative neutron excess parameter, ki,2 - are constants whose values are different for the isotopic and isotonic dependencies, and A,Z,N - are the number of protons and neutron in the nucleus.