Atom's Nuclear Structure and the Periodic Table of the Chemical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -
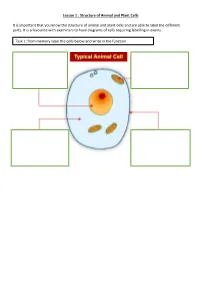
Lesson 1 : Structure of Animal and Plant Cells
Lesson 1 : Structure of Animal and Plant Cells It is important that you know the structure of animal and plant cells and are able to label the different parts. It is a favourite with examiners to have diagrams of cells requiring labelling in exams. Task 1: from memory label the cells below and write in the function Check your answers: There are many similarities and differences between animal and plant cells. Make sure you know these. Similarities Differences 1. Have a nucleus 1. Plant cells have a cellulose cell wall 2. Have a cytoplasm 2. Plant cells have a vacuole containing cell sap 3. Have a cell 3. Plant cells have chloroplast membrane 4. Contain 4. Many plant cells have a box-like shape whilst animal cell shape varies mitochondria 5. Plant cells have the nucleus to the side of the cell, animal cells have a nucleus in 5. Contain ribosomes the middle Task 2: Complete the sentences by filling in the gaps. Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. Check your answers: Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. -

Physics of Superheavy Elements Kouichi Hagino
Frontiers in Science II 2013.11.6 Physics of superheavy elements Kouichi Hagino Nuclear Theory Group, Department of Physics, Tohoku University What is nuclear physics? What are superheavy elements? How to create superheavy elements? What are chemical properties of superheavy elements? Introduction: atoms and atomic nuclei What would you see if you magnified the dog? ~ 50 cm Introduction: atoms and atomic nuclei cells ~ 50 cm ~ m = 10-6 m Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ m = 10-6 m ~ 10 m atom All things are made of atoms. ~ 10-10 m All things are made of atoms. • Thales, Democritus (ancient Greek) • Dalton (chemist, 19th century) • Boltzmann(19th century) • Einstein (1905) ~ 10-10 m STM image (surface physics group, Tohoku university) Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ 10 m atom atomic nucleus ~ 10-15 m ~ 10-10 m proton (+e) neutron (no charge) electron cloud (-e) Neutral atoms: # of protons = # of electrons Chemical properties of atoms # of electrons Mp ~ Mn ~ 2000 Me the mass of atom ~ the mass of nucleus Periodic table of chemical elements tabular arrangement of chemical elements based on the atomic numbers (= # of electrons = # of protons) What are we made of ? oxygen 43 kg cerium 40 mg gallium 0.7 mg carbon 16 kg barium 22 mg tellurium 0.7 mg hydrogen 7 kg iodine 20 mg yttrium 0.6 mg nitrogen 1.8 kg tin 20 mg bismuth 0.5 mg calcium 1.0 kg titanium 20 mg thallium 0.5 mg phosphorus 780 g boron 18 mg indium 0.4 mg potassium 140 g nickel 15 mg gold 0.2 mg sulphur 140 g selenium -

Section 29-4: the Chart of the Nuclides
29-4 The Chart of the Nuclides A nuclide is an atom that is characterized by what is in its nucleus. In other words, it is characterized by the number of protons it has, and by the number of neutrons it has. Figure 29.2 shows the chart of the nuclides, which plots, for stable and radioactive nuclides, the value of Z (the atomic number), on the vertical axis, and the value of N (the number of neutrons) on the horizontal axis. If you look at a horizontal line through the chart, you will find nuclides that all have the same number of protons, but a different number of neutrons. These are all nuclides of the same element, and are known as isotopes (equal proton number, different neutron number). On a vertical line through the chart, the nuclides all have the same number of neutrons, but a different number of protons. These nuclides are known as isotones (equal neutron number, different proton number). It is interesting to think about how the various decay processes play a role in the chart of the nuclides. Only the nuclides shown in black are stable. The stable nuclides give the chart a line of stability that goes from the bottom left toward the upper right, curving down and away from the Z = N line as you move toward higher N values. There are many more nuclides that are shown on the chart but which are not stable - these nuclides decay by means of a radioactive decay process. In general, the dominant decay process for a particular nuclide is the process that produces a nucleus closer to the line of stability than where it started. -

Module01 Nuclear Physics and Reactor Theory
Module I Nuclear physics and reactor theory International Atomic Energy Agency, May 2015 v1.0 Background In 1991, the General Conference (GC) in its resolution RES/552 requested the Director General to prepare 'a comprehensive proposal for education and training in both radiation protection and in nuclear safety' for consideration by the following GC in 1992. In 1992, the proposal was made by the Secretariat and after considering this proposal the General Conference requested the Director General to prepare a report on a possible programme of activities on education and training in radiological protection and nuclear safety in its resolution RES1584. In response to this request and as a first step, the Secretariat prepared a Standard Syllabus for the Post- graduate Educational Course in Radiation Protection. Subsequently, planning of specialised training courses and workshops in different areas of Standard Syllabus were also made. A similar approach was taken to develop basic professional training in nuclear safety. In January 1997, Programme Performance Assessment System (PPAS) recommended the preparation of a standard syllabus for nuclear safety based on Agency Safely Standard Series Documents and any other internationally accepted practices. A draft Standard Syllabus for Basic Professional Training Course in Nuclear Safety (BPTC) was prepared by a group of consultants in November 1997 and the syllabus was finalised in July 1998 in the second consultants meeting. The Basic Professional Training Course on Nuclear Safety was offered for the first time at the end of 1999, in English, in Saclay, France, in cooperation with Institut National des Sciences et Techniques Nucleaires/Commissariat a l'Energie Atomique (INSTN/CEA). -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Isotonic and Isotopic Dependence of the Radiative Neutron Capture Cross-Section on the Neutron Excess
ISOTONIC AND ISOTOPIC DEPENDENCE OF THE RADIATIVE NEUTRON CAPTURE CROSS-SECTION ON THE NEUTRON EXCESS Yu. N. Trofimov V.G. Khlopin Radium Institute, Leningrad Abstract The radiative neutron capture cross-section of nuclei has been derived as a function of neutron excess on the basis of the exponential dependence of the cross-section on the reaction energy. It is shown that unknown cross-sections of stable and radioactive nuclei may be evaluated by using the isotonic and isotopic dependence together with available reference cross-section measurements. Data on fast neutron radiative capture (P3) cross-sections for medium and heavy mass nuclei are at the present time fragmentary and in some cases highly contradictory. This is particularly true of naU [1] where the error of the evaluated cross-section is 50%. The present study is a sequel to the work initiated earlier [2] on the systematic dependence of the P3 neutron cross-section on the isotope mass number, an,r(A). On the basis of the systematics of the P3 neutron cross-sections it is possible: - to reject contradictory or questionable data, and - to predict cross-section values of nuclei which are difficult to measure, including those nuclei which are radioactive. On the basis of the evaluation of P3 neutron cross-sections for nuclei of given parity (three parity groups are taken into consideration here: N and Z even-even, even-odd and odd-even nuclei), the isotopic and isotonic dependence of the cross- section can be represented by o. =K1exp(-K2 a) (1) where a - is the P3 neutron cross-section, or-(N-Z)/A - is the relative neutron excess parameter, ki,2 - are constants whose values are different for the isotopic and isotonic dependencies, and A,Z,N - are the number of protons and neutron in the nucleus. -

Nuclear Mass and Stability
CHAPTER 3 Nuclear Mass and Stability Contents 3.1. Patterns of nuclear stability 41 3.2. Neutron to proton ratio 43 3.3. Mass defect 45 3.4. Binding energy 47 3.5. Nuclear radius 48 3.6. Semiempirical mass equation 50 3.7. Valley of $-stability 51 3.8. The missing elements: 43Tc and 61Pm 53 3.8.1. Promethium 53 3.8.2. Technetium 54 3.9. Other modes of instability 56 3.10. Exercises 56 3.11. Literature 57 3.1. Patterns of nuclear stability There are approximately 275 different nuclei which have shown no evidence of radioactive decay and, hence, are said to be stable with respect to radioactive decay. When these nuclei are compared for their constituent nucleons, we find that approximately 60% of them have both an even number of protons and an even number of neutrons (even-even nuclei). The remaining 40% are about equally divided between those that have an even number of protons and an odd number of neutrons (even-odd nuclei) and those with an odd number of protons and an even number of neutrons (odd-even nuclei). There are only 5 stable nuclei known which have both 2 6 10 14 an odd number of protons and odd number of neutrons (odd-odd nuclei); 1H, 3Li, 5B, 7N, and 50 23V. It is significant that the first stable odd-odd nuclei are abundant in the very light elements 2 (the low abundance of 1H has a special explanation, see Ch. 17). The last nuclide is found in low isotopic abundance (0.25%) and we cannot be certain that this nuclide is not unstable to radioactive decay with extremely long half-life. -

Karlsruhe Nuclide Chart – New 10Th Edition 2018
EPJ Nuclear Sci. Technol. 5, 6 (2019) Nuclear © Sciences Z. Sóti et al., published by EDP Sciences, 2019 & Technologies https://doi.org/10.1051/epjn/2019004 Available online at: https://www.epj-n.org REGULAR ARTICLE Karlsruhe Nuclide Chart – New 10th edition 2018 Zsolt Sóti1,*, Joseph Magill2, and Raymond Dreher2 1 European Commission, Joint Research Centre (JRC), PO Box 2340, 76125 Karlsruhe, Germany 2 Nucleonica GmbH, Magdeburger Str. 2, 76139 Karlsruhe, Germany Received: 20 November 2018 / Received in final form: 12 February 2019 / Accepted: 2 April 2019 Abstract. Obtaining nuclear data is an international activity with new and updated data constantly being determined by thousands of scientists at major research centres worldwide. Because of the large amounts of data generated and the formats used to store these data, the field of nuclear data is highly specialised. To make the most important key data more accessible to a wider audience, nuclide charts have been developed. In this article, we present the scientific highlights of the new 10th Edition of the Karlsruhe Nuclide Chart. The main focus of this Chart is to provide structured, accurate information on the half-lives and decay modes, as well as energies of the emitted radiation for over 4000 experimentally observed ground states and isomer nuclides to an interdisciplinary audience. 1 Introduction The new 10th Edition of the Karlsruhe Nuclide Chart contains nuclear data on 4040 experimentally observed The United Nations has designated 2019 as the interna- nuclide ground states and isomers. New data on tional year of the periodic table of chemical elements, 696 nuclides has been included together with information recognizing it as an instrument “central to linking cultural, on 47 new nuclides. -

Periodic Table of the Elements Vs. Nuclide Chart 2) Nuclear Stability
2) Nuclear Stability and nuclear radiation (2) Periodic Table of the Elements vs. Nuclide Chart Approx. 115 different chemical elements ⇒ Periodic Table of Elements Elements with naturally occuring radioactive isotopes (selection) Elements without stable isotopes 7 2) Nuclear Stability and nuclear radiation (3) Periodic Table of the Elements vs. Nuclide Chart Approx. 2800 different nuclides ⇒ Chart of nuclides 8 1 2) Nuclear Stability and nuclear radiation (4) Dependence of the nuclear stability on the composition of the nuclei Line of ß--stability 9 2) Nuclear Stability and nuclear radiation (5) Proton/neutron number Statistical ResuǗ checking all stable nuclides Number of Number of Probability protons Neutrons Even Even Very common, 158 nuclei Even Odd Common, 53 nuclei Odd Even Common, 50 nuclei Odd Odd Rare , only 6 nuclei 10 2 The Nuclear Binding energy andnuclide masses The theory of proton/neutron orbitals Isobaric nuclides Instable Stable Instable ß 2) Nuclear Stability and nuclear radiation (7) - decay ß Nuclear Binding Energy per nucleon in MeV/u 2) Nuclear Stability and nuclear radiation (6) 12 X andassumed nucleone orbitals Mass number o/e, e/o nuclides o/e, e/o e/e nuclides + decay 11 12 3 2) Nuclear Stability and nuclear radiation (8) The Nuclear Binding energy and nuclide masses Remember! mass of a proton: 1.67252 x 10-27 kg mass of a neutron: 1.67482 x 10-27 kg The mass of an atomic nucleus is always less than that of the sum of its components. Mass of a nuclide: M = Z Mproton + N Mneutron - δM where δM is the mass defect