Pharmacological Investigation of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -

Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy
Japan. J. Pharmacol. 35, 319-326 (1984) 319 Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy Shigeru IKEDA, Hiroshi TAMAOKI, Masuo AKAHANE and Yoshifumi NEBASHI Central ResearchLaboratories, Kissei Pharmaceutical Co., Ltd. 19-48 Yoshino,Matsumoto 399-65, Japan Accepted April7, 1984 Abstract•\Ritodrine hydrochloride (ritodrine) is a beta2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10-9-1 0-6 M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100-1,000 times less than that of iso- proterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol> ritodrine>isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with pro- pranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors. -
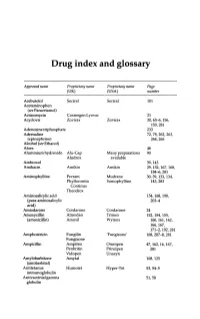
Drug Index and Glossary
Drug index and glossary Approved name Proprietary name Proprietary name Page (UK) (USA) number Acebutolol Sectral Sectral 101 Acetaminophen (see Paracetamol) Actinomycin Cosmegen Lyovac 21 Acyclovir Zovirax Zovirax 30, 65-6, 156, 159,281 Adenosine triphosphate 233 Adrenaline 72, 78, 262, 263, (epinephrine) 264,265 Alcohol (see Ethanol) Aloes 48 Aluminium hydroxide Alu-Cap Many preparations 90 Aludrox available Ambroxol 39,143 Amikacin Amikin Amikin 29,152,167,168, 184-6,281 Aminophylline Pecram Mudrane 30,39,133,134, Phyllocontin Somophylline 143,283 Continus Theodrox Aminosalicylic acid 154, 168, 198, (para-aminosalicylic 203-4 acid) Amiodarone Cordarone Cordarone 24 Amoxycillin Almodan Trlmox 152, 154, 155, (amoxicillin) Amoxil Wymox 160, 161, 162, 166,167, 171-2, 192, 281 Amphotericin Fungilin 'Fungizone' 168, 207--S, 281 Fungizone Ampicillin Ampifen Omnipen 47,162,16,167, Penbritin Principen 281 Vidopen Unasyn Amylobarbitone Amytal 108,125 (amobarbital) Antitetanus Humotet Hyper-Tet 53,54-5 immunoglobulin Antivaccinial gamma 51,58 globulin Drug index and glossary 289 Approved name Proprietary name Proprietary name Page (UK) (USA) number Antivaricella-zoster 65 immunoglobulin Apomorphine 97 Arnica 278 Ascorbic acid Redoxon Many preparations 70,75--6 available Aspirin Many preparations Many preparations 23,48,99,100, available available 141,272-3,276 Atebrin 12 (Atabrine, Medacrine, Quinacrine) Atenolol Tenormin Tenormin 101,120 Atropine Many combined Many combined 48,49,96,222, preparations preparations 234,250,26S-9 available available -

Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides
DRUGS Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides (Generic Examples: Gentamycin; Tobramycin; Amikacin) Amoxicillin Amphotericin B Ampicillin Azithromycin (A Special Macrolide) Ceftriaxone (Prototype 3rd Generation Cephalosporin) Chloramphenicol Chloroquine, Quinine and Mefloquine Ciprofloxacin (Prototype Fluoroquinolone) Clindamycin Demeclocycline (Another Tetracycline) Doxycycline (Prototype Tetracycline) Erythromycin (Prototype Macrolide) Indinavir Isoniazid Ketoconazole Metronidazole Nafcillin (Beta-Lactamase Resistant Penicillin) Praziquantel Rifampin Trimethoprim + Sulfamethoxazole Vancomycin Zidovudine Autonomic Drugs : Atropine Bethanechol Clonidine Cocaine Dobutamine Edrophonium Epinephrine Isoproteronol Labetalol (also Carvedilol) Methylphenidate Metoprolol Phenoxybenzamine Phentolamine Phenylepherine Physostigmine Pilocarpine Pindolol (also Acebutolol) Prazosin Propranolol Reserpine Ritodrine Blood D/O Drugs: Heparin Streptokinase TPA (Tissue Plasminogen Activator) Warfarin Cancer Drugs: 5-Fluoruracil + Folic Acid Bleomycin Busulfan Cisplatin Colchicine Cyclophoshamide Cyclosporine Dacarbazine Doxorubicin = Adriamycin Melphalan Methotrexate Vinchristine Cardiovascular Drugs: Amiodarone Captopril and Enalapril Digoxin Diltiazem Hydralazine Lidocaine Losartan Nifedipine Nitroglycerin and Isosorbide Dinitrate Nitroprusside Procainamide Quinidine Verapamil CNS Drugs: Alprazolam (Another Benzodiazepine) Desipramine (Secondary TCA) Diazepam (Prototype -
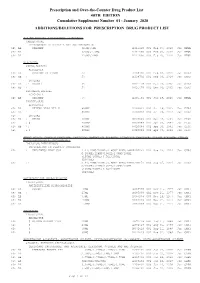
January 2020: Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 01 : January 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> AA XIROMED 325MG;5MG A 211690 001 Feb 07, 2020 Jan NEWA >A> AA 325MG;7.5MG A 211690 002 Feb 07, 2020 Jan NEWA >A> AA 325MG;10MG A 211690 003 Feb 07, 2020 Jan NEWA ACYCLOVIR CREAM;TOPICAL ACYCLOVIR >D> AB PERRIGO UK FINCO 5% A 208702 001 Feb 04, 2019 Jan CHRS >A> AB ! 5% A 208702 001 Feb 04, 2019 Jan CHRS ZOVIRAX >D> AB +! BAUSCH 5% N 021478 001 Dec 30, 2002 Jan CHRS >A> AB + 5% N 021478 001 Dec 30, 2002 Jan CHRS OINTMENT;TOPICAL ACYCLOVIR >A> AB XIROMED 5% A 201501 001 Jan 29, 2020 Jan NEWA TABLET;ORAL ACYCLOVIR >D> AB HETERO LABS LTD V 800MG A 203834 002 Oct 29, 2013 Jan CHRS >A> AB ! 800MG A 203834 002 Oct 29, 2013 Jan CHRS >D> ZOVIRAX >D> AB + MYLAN 400MG N 020089 001 Apr 30, 1991 Jan DISC >A> + @ 400MG N 020089 001 Apr 30, 1991 Jan DISC >D> AB +! 800MG N 020089 002 Apr 30, 1991 Jan DISC >A> + @ 800MG N 020089 002 Apr 30, 1991 Jan DISC AMINO ACIDS; CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM SULFATE; POTASSIUM CHLORIDE; SODIUM ACETATE; SODIUM GLYCEROPHOSPHATE; SOYBEAN OIL EMULSION;INTRAVENOUS PERIKABIVEN IN PLASTIC CONTAINER >D> + FRESENIUS KABI USA 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML ;105MG/100ML;3.5GM/100ML (2400ML) >A> +! 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML -

Adrenoceptor Agonists on Spontaneous Contractions of Human Nonpregnant Myometrium
PRACE ORYGINALNE Ginekol Pol. 2011, 82, 918-924 ginekologia Differences in the effects ofβ 2- and β3- adrenoceptor agonists on spontaneous contractions of human nonpregnant myometrium Odmienny wpływ agonistów receptorów β2- i β3-adrenergicznych na spontaniczne skurcze myometrium kobiet nieciężarnych Pędzińska-Betiuk Anna1*, Modzelewska Beata1, Jóźwik Marcin2, Jóźwik Maciej3, Kostrzewska Anna1 1 Department of Biophysics, Medical University of Białystok, Białystok, Poland 2 Department of Gynecology and Obstetrics, Kliniken Nordoberpfalz, Akademisches Lehrkrankenhaus der Universität Regensburg, Weiden, Germany 3 Department of Gynecology, Medical University of Białystok, Białystok, Poland * Current address: Department of Experimental Physiology and Pathophysiology, Medical University of Białystok, Białystok, Poland Abstract Objective: This study aimed to compare the relaxant properties of BRL 37344 with β2-adrenoceptors agonist ritodrine on the contractility of human nonpregnant myometrium. Material and methods: The activity of myometrial strips mounted in an organ bath was recorded under isometric conditions using force transducers with digital output. Contractility before and after cumulative additions of both uterorelaxants and with preincubation with β-adrenoceptor antagonists bupranolol, propranolol, and butoxamine were studied. Results: Both BRL 37344 (10-10 – 10-4 mol/L) and ritodrine (10-10 – 10-5 mol/L) decreased the area under curve, or AUC, value (logIC50 -6.45 ± 0.18 and -8.71 ± 0.35, respectively), and the degree of inhibition of spontaneous contractile activity was similar (< 30%). However, BRL 37344 decreased the mean frequency of contractions, whereas ritodrine decreased the mean amplitude of contractions. The inhibition of contractions by BRL 37344 was partially antagonized by bupranolol and propranolol, but not with butoxamine. The inhibition by ritodrine was counteracted by all these antagonists. -

List Item Short-Acting Beta-Agonists Article-31 Referral
Annex II Scientific conclusions and grounds for revocation or variation as applicable to the terms of the marketing authorisations and detailed explanation for the differences from the PRAC recommendation 19 Scientific conclusions and grounds for revocation or variation as applicable to the terms of the marketing authorisations and detailed explanation for the differences from the PRAC recommendation The CMDh considered the below PRAC recommendation dated 5 September 2013 with regards to the terbutaline, salbutamol, hexoprenaline, ritodrine, fenoterol and isoxsuprine containing medicinal products: 1. Overall summary of the scientific evaluation of terbutaline, salbutamol, hexoprenaline, ritodrine, fenoterol and isoxsuprine containing medicinal products by PRAC (see Annex I) On 27 November 2012, further to evaluation of data resulting from pharmacovigilance activities, Hungary informed the European Medicines Agency, pursuant to Article 31 of Directive 2001/83/EC, of their consideration that the risk-benefit balance of short-acting beta-agonists (SABAs) containing medicinal products authorised in obstetric indications has become unfavourable, taking into account the cardiovascular events reported. Hungary considered it was in the interest of the Union to refer the matter to the PRAC and expressed concerns with regards to the posology and warnings reflected in the product information. The short-acting beta-agonists (SABAs) (also known as beta-mimetics), salbutamol, terbutaline, fenoterol, ritodrine, hexoprenaline and isoxsuprine are all nationally authorised and have been on the market within the EU since the 1960s. Authorised obstetric indications for SABAs differ across Member States. The authorised obstetric indications include partus prematurus, tocolysis (for some products use is restricted to particular weeks of gestation but for others no specific gestation period is specified), external cephalic version (ECV), and hyper-uterine contractility. -

Medications to Avoid in Long QT Syndrome
Jackie Crawford Cardiac Inherited Disease Co-ordinator C/- Paediatric Cardiology; Level 3 Starship; Auckland City Hospital Private Bag 92024; Auckland Cardiac Inherited Disease Registry Phone: (09) 3074949 ext 23634 www.cidg.org Fax: (09) 6309877 Email: [email protected] Medications to be avoided, or requiring special caution, in people with Long QT syndrome This list includes medications which prolong the QT interval and is meant as a guide for people with Long QT syndrome, or acquired long QT interval from heart muscle disease, and their parents or guardians. It should not be seen as all inclusive. Those prescribing any medication to someone with Long QT syndrome should always check the drug specifications and contra-indications. The list has been compiled by review of publications and/or drug advice sheets provided with medications. Check also www.SADS.org and www.Torsades .org. Antibiotics Erythromycin, Clarithromycin, Gatifloxacin, levofloxacin, Moxifloxacin Sulfamethoxazole-trimethoprim (Septrin/Bactrim), Spiramycin, Pentamidine Antihistamines Terfenadine, Astemizole, Diphenhydramine, (These are particularly to be avoided (even in normal subjects) in combination with Erythromycin or grapefruit juice or the antifungals ketoconazole, miconazole, fluconazole or itraconazole) [Antihistamines that may be used safely are loratidine, cetirizine and fexofenadine, and phenergan] Appetite suppressants Fenfluramine, phentermine, Sibutramine Asthma treatments The Beta-2 agonists (e.g. Terbutaline, Salbutamol, Salmeterol) both work against -

Assessment Report for Short Acting Beta Agonists (Sabas) Containing Medicinal Products Authorised in Obstetric Indications
23 October 2013 EMA/664276/2013 Assessment report for Short Acting Beta Agonists (SABAs) containing medicinal products authorised in obstetric indications Procedure under Article 31 of Directive 2001/83/EC INN/active substance: terbutaline, salbutamol, hexoprenaline, ritodrine, fenoterol, isoxsuprine Procedure number: EMEA/H/A-31/1347 Assessment Report as adopted by PRAC with all the information of a confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 3 2. Scientific discussion ................................................................................ 3 2.1. Clinical aspects .................................................................................................... 4 2.1.1. Safety .............................................................................................................. 4 2.1.2. Efficacy ............................................................................................................ 8 2.2. Risk minimisation activities .................................................................................. 13 2.3. Product information ........................................................................................... -

ROBINUL® (Glycopyrronium Bromide (Glycopyrrolate) Injection)
NEW ZEALAND DATA SHEET 1 PRODUCT NAME ROBINUL® (Glycopyrronium bromide (glycopyrrolate) Injection) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 1 mL ampoule contains Glycopyrronium bromide (glycopyrrolate) 0.2 mg, Water for Injections q.s., Sodium Chloride 9 mg and Sodium hydroxide/Hydrochloric acid (for pH adjustment) Glycopyrronium bromide (glycopyrrolate) is a white, odourless, crystalline powder with a bitter taste. It is a quaternary ammonium compound. Glycopyrronium bromide (glycopyrrolate) is chemically designated as 3- (alpha-cyclopentylmandeloyloxy)-1, 1-dimethylpyrrolidinium bromide and has a molecular weight of 398.34. It is soluble in water and alcohol and practically insoluble in chloroform and ether. ROBINUL is a clear, colourless sterile solution with a pH of 2.5 - 4.0. The chemical name(s) for Glycopyrronium bromide (glycopyrrolate) is : Pyrrolidinium, 3- [(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl- bromide. 3-Hydroxy-1,1-dimethylpyrrolidinium bromide α-cyclopentylmandelate Molecular formula: C19H28 BrN03 Molecular Mass: 398.33 CAS: [596-51-0] . 3 PHARMACEUTICAL FORM Injection 4 CLINICAL PARTICULARS 4.1 Therapeutic indications In Anaesthesia: ROBINUL is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial and pharyngeal secretions; to reduce the volume and free acidity of gastric secretions, and to block cardiac vagal inhibitory reflexes during induction of anaesthesia and intubation when indicated. ROBINUL Injectable may be used intraoperatively to counteract drug-induced or -

Toxic and Drug-Induced Changes of the Electrocardiogram
15 Toxic and Drug-Induced Changes of the Electrocardiogram Catalina Lionte, Cristina Bologa and Laurentiu Sorodoc ”Gr.T.Popa” University of Medicine and Pharmacy, Iasi, Romania 1. Introduction There are numerous toxins and drugs that can cause, in overdose, electrocardiogram (ECG) changes, even in patients without history of cardiac pathology. The diagnosis and management of patients with an abnormal ECG encountered in a specific toxicity can challenge experienced physicians. One must have serious knowledge of basic cardiac physiology, in order to understand the ECG changes associated with various drugs and toxins. The main mechanisms involved include membrane – depressant action (sodium channel blockers, slow calcium channel blockers, outward potassium (K+) channel blockers, and sodium-potassium adenosine-triphosphatase blockers), and action on autonomic nervous system and its sites of cardiovascular action (beta-adrenergic blockers and other sympathetic-inhibitors, sympathomimetic, anticholinergic and cholinomimetic substances). Many toxins and medications have actions that involve more than one of these mechanisms, including hypoxia, electrolyte and metabolic imbalances, and thus may result in a combination of electrocardiographic changes. In resting state, the myocardial cell membrane is impermeable to positively charged sodium ions (Na+). The Na+/K+ ATPase maintains a negative electric potential of approximately 90 mV in the myocyte. The rapid opening of Na+ channels and massive Na+ influx (phase 0 of action potential) explains depolarization of the cardiac cell membrane (fig.1), causing the rapid upstroke of the cardiac action potential, which is conducted through the ventricles and is expressed as the QRS complex of the ECG. The closure of Na+ channels and the transient opening of Ito K+ efflux channels (phase 1) mark the peak of the action potential. -

Evidence of Atypical Fl-Adrenoceptors in Rat Colon
Br. J. Pharmacol. (1990), 100, 831-839 (C.") Macmillan Press Ltd, 1990 In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical fl-adrenoceptors in rat colon 'Alberto Bianchetti & Luciano Manara Research Center Sanofi-Midy S.p.A., Via G.B. Piranesi, 38, 20137 Milan, Italy 1 The new compounds phenylethanolaminotetralines (PEAT), unlike the reference ,B-adrenoceptor agon- ists isoprenaline (Iso), ritodrine (Ri) and salbutamol (Sal), produced half-maximal inhibition of sponta- neous motility of rat isolated proximal colon at substantially lower concentrations (EC50 2.7-30nM) than those inducing f2-adrenoceptor-mediated responses (relaxation of guinea-pig isolated trachea and rat uterus) and had virtually no chronotropic action (EC50 > 3 x 10- 5 M) on the guinea-pig isolated atrium (a f1-adrenoceptor-mediated response). 2 The nonselective f-adrenoceptor antagonists alprenolol and propranolol prevented the inhibition of rat colon motility by the PEAT with low and different potencies (pA2 values around 7.5 and 6.5 respectively). Conversely alprenolol and propranolol had a higher and similar potency (pA2 values around 9.0) in preventing typical f6l- or f2-responses (increase in atrial frequency by Iso or tracheal relaxation by Ri or Sal). 3 The selective 8-adrenoceptor antagonists CGP 20712A (#k) and ICI 118,551 (82) either alone or in combination, did not prevent rat colon motility inhibition by the representative PEAT SR 5861 IA, which was also fully resistant to a-adrenoceptor, acetylcholine, dopamine, histamine, opioid and 5- hydroxytryptamine antagonists. 4 These results indicate that the PEAT are a new class of fi-adrenoceptor agonists and suggest that their preferential intestinal action may be accounted for by selectivity for atypical fl-adrenoceptors, abundant in the rat colon and distinct from the currently recognized 1i and fl2 subtypes.