Tocolytic Therapy a Meta-Analysis and Decision Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

Cord Prolapse
CLINICAL PRACTICE GUIDELINE CORD PROLAPSE CLINICAL PRACTICE GUIDELINE CORD PROLAPSE Institute of Obstetricians and Gynaecologists, Royal College of Physicians of Ireland and the Clinical Strategy and Programmes Division, Health Service Executive Version: 1.0 Publication date: March 2015 Guideline No: 35 Revision date: March 2017 1 CLINICAL PRACTICE GUIDELINE CORD PROLAPSE Table of Contents 1. Revision History ................................................................................ 3 2. Key Recommendations ....................................................................... 3 3. Purpose and Scope ............................................................................ 3 4. Background and Introduction .............................................................. 4 5. Methodology ..................................................................................... 4 6. Clinical Guidelines on Cord Prolapse…… ................................................ 5 7. Hospital Equipment and Facilities ....................................................... 11 8. References ...................................................................................... 11 9. Implementation Strategy .................................................................. 14 10. Qualifying Statement ....................................................................... 14 11. Appendices ..................................................................................... 15 2 CLINICAL PRACTICE GUIDELINE CORD PROLAPSE 1. Revision History Version No. -

Tractocile, Atosiban
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Tractocile 6.75 mg/0.9 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial of 0.9 ml solution contains 6.75 mg atosiban (as acetate). For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection (injection). Clear, colourless solution without particles. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Tractocile is indicated to delay imminent pre-term birth in pregnant adult women with: regular uterine contractions of at least 30 seconds duration at a rate of 4 per 30 minutes a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of 50% a gestational age from 24 until 33 completed weeks a normal foetal heart rate 4.2 Posology and method of administration Posology Treatment with Tractocile should be initiated and maintained by a physician experienced in the treatment of pre-term labour. Tractocile is administered intravenously in three successive stages: an initial bolus dose (6.75 mg), performed with Tractocile 6.75 mg/0.9 ml solution for injection, immediately followed by a continuous high dose infusion (loading infusion 300 micrograms/min) of Tractocile 37.5 mg/5 ml concentrate for solution for infusion during three hours, followed by a lower dose of Tractocile 37.5 mg/5 ml concentrate for solution for infusion (subsequent infusion 100 micrograms/min) up to 45 hours. The duration of the treatment should not exceed 48 hours. The total dose given during a full course of Tractocile therapy should preferably not exceed 330.75 mg of atosiban. -

Tocolytics Used As Adjunctive Therapy at the Time of Cerclage Placement: a Systematic Review
Journal of Perinatology (2015) 35, 561–565 © 2015 Nature America, Inc. All rights reserved 0743-8346/15 www.nature.com/jp ORIGINAL ARTICLE Tocolytics used as adjunctive therapy at the time of cerclage placement: a systematic review J Smith1,2 and EA DeFranco3,4 OBJECTIVE: To review the published literature on whether the use of empiric perioperative tocolytic medications could provide additional benefit when used in combination with cerclage. STUDY DESIGN: Systematic review of published medical literature reporting the efficacy of empiric tocolytics used as a perioperative adjunct to vaginal cerclage in high-risk patients. A PubMed search without date criteria of various tocolytics and cerclage yielded 42 studies. Review articles were excluded, as were reports of abdominal cerclage, emergent cerclage, or cerclage for the purpose of delayed interval delivery in twin gestations. RESULT: Only five publications on the topic of perioperative tocolytic use at the time of history or ultrasound-indicated vaginal cerclage placement were identified. These included zero clinical trials, three retrospective cohort studies, one case series and one case report. Only one cohort study compared cerclage with indomethacin and cerclage without indomethacin and suggested no difference between the groups. The other two published cohort studies had no referent group who received cerclage without tocolysis. One case series and one case report were also published reporting cerclage with empiric beta-mimetic and progesterone adjunctive therapy. CONCLUSION: There is a paucity of published data on the topic of adjunctive perioperative tocolytics with cerclage. Adequately powered clinical trials on perioperative use of tocolysis with cerclage compared with a standard cerclage placement alone are needed to establish efficacy. -

Pharmaceutical Services Division and the Clinical Research Centre Ministry of Health Malaysia
A publication of the PHARMACEUTICAL SERVICES DIVISION AND THE CLINICAL RESEARCH CENTRE MINISTRY OF HEALTH MALAYSIA MALAYSIAN STATISTICS ON MEDICINES 2008 Edited by: Lian L.M., Kamarudin A., Siti Fauziah A., Nik Nor Aklima N.O., Norazida A.R. With contributions from: Hafizh A.A., Lim J.Y., Hoo L.P., Faridah Aryani M.Y., Sheamini S., Rosliza L., Fatimah A.R., Nour Hanah O., Rosaida M.S., Muhammad Radzi A.H., Raman M., Tee H.P., Ooi B.P., Shamsiah S., Tan H.P.M., Jayaram M., Masni M., Sri Wahyu T., Muhammad Yazid J., Norafidah I., Nurkhodrulnada M.L., Letchumanan G.R.R., Mastura I., Yong S.L., Mohamed Noor R., Daphne G., Kamarudin A., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Omar I., Zoriah A., Fong Y.Y.A., Nusaibah A.R., Feisul Idzwan M., Ghazali A.K., Hooi L.S., Khoo E.M., Sunita B., Nurul Suhaida B.,Wan Azman W.A., Liew H.B., Kong S.H., Haarathi C., Nirmala J., Sim K.H., Azura M.A., Asmah J., Chan L.C., Choon S.E., Chang S.Y., Roshidah B., Ravindran J., Nik Mohd Nasri N.I., Ghazali I., Wan Abu Bakar Y., Wan Hamilton W.H., Ravichandran J., Zaridah S., Wan Zahanim W.Y., Kannappan P., Intan Shafina S., Tan A.L., Rohan Malek J., Selvalingam S., Lei C.M.C., Ching S.L., Zanariah H., Lim P.C., Hong Y.H.J., Tan T.B.A., Sim L.H.B, Long K.N., Sameerah S.A.R., Lai M.L.J., Rahela A.K., Azura D., Ibtisam M.N., Voon F.K., Nor Saleha I.T., Tajunisah M.E., Wan Nazuha W.R., Wong H.S., Rosnawati Y., Ong S.G., Syazzana D., Puteri Juanita Z., Mohd. -

Indomethacin in Pregnancy: Applications and Safety
Original Article 175 Indomethacin in Pregnancy: Applications and Safety Gael Abou-Ghannam, M.D. 1 Ihab M. Usta, M.D. 1 Anwar H. Nassar, M.D. 1 1 Department of Obstetrics and Gynecology, American University of Address for correspondence and reprint requests Anwar H. Nassar, Beirut Medical Center, Hamra, Beirut, Lebanon M.D., American University of Beirut Medical Center, P.O. Box 113-6044/B36, Hamra 110 32090, Beirut, Lebanon (e-mail: [email protected]). Am J Perinatol 2012;29:175–186. Abstract Preterm labor (PTL) is a major cause of neonatal morbidity and mortality worldwide. Among the available tocolytics, indomethacin, a prostaglandin synthetase inhibitor, has been in use since the 1970s. Recent studies have suggested that prostaglandin synthetase inhibitors are superior to other tocolytics in delaying delivery for 48 hours and 7 days. However, increased neonatal complications including oligohydramnios, Keywords renal failure, necrotizing enterocolitis, intraventricular hemorrhage, and closure of the ► indomethacin patent ductus arteriosus have been reported with the use of indomethacin. Indometh- ► tocolysis acin has been also used in women with short cervices as well as in those with idiopathic ► preterm labor polyhydramnios. This article describes the mechanism of action of indomethacin and its ► short cervix clinical applications as a tocolytic agent in women with PTL and cerclage and its use in ► polyhydramnios the context of polyhydramnios. The fetal and neonatal side effects of this drug are also ► fetal side effects summarized and guidelines for its use are proposed. Preterm labor (PTL) is a major cause of neonatal morbidity in women with PTL and cerclage and its use in the context of and mortality worldwide.1 Care of premature infants has polyhydramnios. -

Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy
Japan. J. Pharmacol. 35, 319-326 (1984) 319 Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy Shigeru IKEDA, Hiroshi TAMAOKI, Masuo AKAHANE and Yoshifumi NEBASHI Central ResearchLaboratories, Kissei Pharmaceutical Co., Ltd. 19-48 Yoshino,Matsumoto 399-65, Japan Accepted April7, 1984 Abstract•\Ritodrine hydrochloride (ritodrine) is a beta2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10-9-1 0-6 M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100-1,000 times less than that of iso- proterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol> ritodrine>isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with pro- pranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors. -
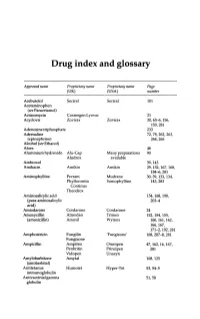
Drug Index and Glossary
Drug index and glossary Approved name Proprietary name Proprietary name Page (UK) (USA) number Acebutolol Sectral Sectral 101 Acetaminophen (see Paracetamol) Actinomycin Cosmegen Lyovac 21 Acyclovir Zovirax Zovirax 30, 65-6, 156, 159,281 Adenosine triphosphate 233 Adrenaline 72, 78, 262, 263, (epinephrine) 264,265 Alcohol (see Ethanol) Aloes 48 Aluminium hydroxide Alu-Cap Many preparations 90 Aludrox available Ambroxol 39,143 Amikacin Amikin Amikin 29,152,167,168, 184-6,281 Aminophylline Pecram Mudrane 30,39,133,134, Phyllocontin Somophylline 143,283 Continus Theodrox Aminosalicylic acid 154, 168, 198, (para-aminosalicylic 203-4 acid) Amiodarone Cordarone Cordarone 24 Amoxycillin Almodan Trlmox 152, 154, 155, (amoxicillin) Amoxil Wymox 160, 161, 162, 166,167, 171-2, 192, 281 Amphotericin Fungilin 'Fungizone' 168, 207--S, 281 Fungizone Ampicillin Ampifen Omnipen 47,162,16,167, Penbritin Principen 281 Vidopen Unasyn Amylobarbitone Amytal 108,125 (amobarbital) Antitetanus Humotet Hyper-Tet 53,54-5 immunoglobulin Antivaccinial gamma 51,58 globulin Drug index and glossary 289 Approved name Proprietary name Proprietary name Page (UK) (USA) number Antivaricella-zoster 65 immunoglobulin Apomorphine 97 Arnica 278 Ascorbic acid Redoxon Many preparations 70,75--6 available Aspirin Many preparations Many preparations 23,48,99,100, available available 141,272-3,276 Atebrin 12 (Atabrine, Medacrine, Quinacrine) Atenolol Tenormin Tenormin 101,120 Atropine Many combined Many combined 48,49,96,222, preparations preparations 234,250,26S-9 available available -

210428Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 210428Orig1s000 OTHER REVIEW(S) DIVISION OF CARDIOVASCULAR AND RENAL PRODUCTS Regulatory Project Manager Overview I. GENERAL INFORMATION NDA: 210428 Drug: Metoprolol Succinate Extended-Release Capsules, 25 mg, 50 mg, 100 mg and 200 mg Class: Beta- Blocker Applicant: Sun Pharmaceutical Industries Limited Proposed Indications: Hypertension, Angina Pectoris & Heart Failure Date of submission: March 30, 2017 PDUFA date: January 30, 2018 II. REVIEW TEAM Office of New Drugs, Office of Drug Evaluation I: Division of Cardiovascular & Renal Product Norman Stockbridge, MD, PhD, Director Mary Ross Southworth, PharmD, Deputy Director for Safety Michael Monteleone, MS, RAC, Assistant Director for Labeling Martina Sahre, PhD, Cross Discipline Team Leader Fortunato Senatore, MD, Clinical Reviewer Albert DeFelice, PhD, Non-Clinical Supervisor Muriel Saulnier, PhD, Non-Clinical Reviewer Edward Fromm, RPh, RAC, Chief, Project Management Staff Maryam Changi, PharmD, Regulatory Project Manager Office of Pharmaceutical Quality: Wendy Wilson-Lee, PhD, Application Technical Lead Grafton Adams, Regulatory Business Process Manager Milton Sloan, PhD, Drug Product Reviewer Rohit Tiwari, PhD, Drug Substance Reviewer Ben Stevens, PhD, Drug Substance Team Leader Kaushal Dave, PhD, Biopharmaceutical Reviewer Wu, Ta-Chin, PhD, Biopharmaceutical Team Leader Viviana Matta, PhD, Facility Reviewer Ruth Moore, PhD, Facility Team Leader Chunsheng Cai, PhD, Process Reviewer NDA 210428 RPM review Page 1 Reference ID: 42132034213643 Labeling/PMC-PMR to Sponsor: November 30, 2017 PDUFA Date: January 30, 2018 (Standard, 10-Month) PDUFA Goal Date: January 30, 2018 Approval letter: January 26, 2018 7. Reviews a) Divisional Memorandum: (January 26, 2018) Dr. Stockbridge indicated his concurrence on Dr. Sahre’s CDTL memo. -

Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides
DRUGS Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides (Generic Examples: Gentamycin; Tobramycin; Amikacin) Amoxicillin Amphotericin B Ampicillin Azithromycin (A Special Macrolide) Ceftriaxone (Prototype 3rd Generation Cephalosporin) Chloramphenicol Chloroquine, Quinine and Mefloquine Ciprofloxacin (Prototype Fluoroquinolone) Clindamycin Demeclocycline (Another Tetracycline) Doxycycline (Prototype Tetracycline) Erythromycin (Prototype Macrolide) Indinavir Isoniazid Ketoconazole Metronidazole Nafcillin (Beta-Lactamase Resistant Penicillin) Praziquantel Rifampin Trimethoprim + Sulfamethoxazole Vancomycin Zidovudine Autonomic Drugs : Atropine Bethanechol Clonidine Cocaine Dobutamine Edrophonium Epinephrine Isoproteronol Labetalol (also Carvedilol) Methylphenidate Metoprolol Phenoxybenzamine Phentolamine Phenylepherine Physostigmine Pilocarpine Pindolol (also Acebutolol) Prazosin Propranolol Reserpine Ritodrine Blood D/O Drugs: Heparin Streptokinase TPA (Tissue Plasminogen Activator) Warfarin Cancer Drugs: 5-Fluoruracil + Folic Acid Bleomycin Busulfan Cisplatin Colchicine Cyclophoshamide Cyclosporine Dacarbazine Doxorubicin = Adriamycin Melphalan Methotrexate Vinchristine Cardiovascular Drugs: Amiodarone Captopril and Enalapril Digoxin Diltiazem Hydralazine Lidocaine Losartan Nifedipine Nitroglycerin and Isosorbide Dinitrate Nitroprusside Procainamide Quinidine Verapamil CNS Drugs: Alprazolam (Another Benzodiazepine) Desipramine (Secondary TCA) Diazepam (Prototype -
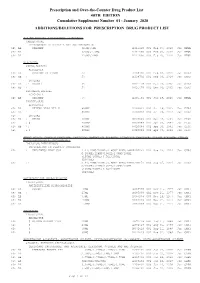
January 2020: Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 01 : January 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> AA XIROMED 325MG;5MG A 211690 001 Feb 07, 2020 Jan NEWA >A> AA 325MG;7.5MG A 211690 002 Feb 07, 2020 Jan NEWA >A> AA 325MG;10MG A 211690 003 Feb 07, 2020 Jan NEWA ACYCLOVIR CREAM;TOPICAL ACYCLOVIR >D> AB PERRIGO UK FINCO 5% A 208702 001 Feb 04, 2019 Jan CHRS >A> AB ! 5% A 208702 001 Feb 04, 2019 Jan CHRS ZOVIRAX >D> AB +! BAUSCH 5% N 021478 001 Dec 30, 2002 Jan CHRS >A> AB + 5% N 021478 001 Dec 30, 2002 Jan CHRS OINTMENT;TOPICAL ACYCLOVIR >A> AB XIROMED 5% A 201501 001 Jan 29, 2020 Jan NEWA TABLET;ORAL ACYCLOVIR >D> AB HETERO LABS LTD V 800MG A 203834 002 Oct 29, 2013 Jan CHRS >A> AB ! 800MG A 203834 002 Oct 29, 2013 Jan CHRS >D> ZOVIRAX >D> AB + MYLAN 400MG N 020089 001 Apr 30, 1991 Jan DISC >A> + @ 400MG N 020089 001 Apr 30, 1991 Jan DISC >D> AB +! 800MG N 020089 002 Apr 30, 1991 Jan DISC >A> + @ 800MG N 020089 002 Apr 30, 1991 Jan DISC AMINO ACIDS; CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM SULFATE; POTASSIUM CHLORIDE; SODIUM ACETATE; SODIUM GLYCEROPHOSPHATE; SOYBEAN OIL EMULSION;INTRAVENOUS PERIKABIVEN IN PLASTIC CONTAINER >D> + FRESENIUS KABI USA 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML ;105MG/100ML;3.5GM/100ML (2400ML) >A> +! 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML