210428Orig1s000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacological Interventions for Promoting Smoking Cessation During Pregnancy (Review)
Pharmacological interventions for promoting smoking cessation during pregnancy (Review) Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 12 http://www.thecochranelibrary.com Pharmacological interventions for promoting smoking cessation during pregnancy (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 3 OBJECTIVES ..................................... 6 METHODS ...................................... 6 RESULTS....................................... 10 Figure1. ..................................... 13 Figure2. ..................................... 14 DISCUSSION ..................................... 17 AUTHORS’CONCLUSIONS . 19 ACKNOWLEDGEMENTS . 19 REFERENCES ..................................... 19 CHARACTERISTICSOFSTUDIES . 26 DATAANDANALYSES. 45 Analysis 1.1. Comparison 1 Nicotine replacement therapy versus control (Primary outcome - efficacy), Outcome 1 Validated cessation in later pregnancy. ....... 46 Analysis 1.2. Comparison 1 Nicotine replacement therapy versus control (Primary outcome - efficacy), Outcome 2 Self- report cessation at 3 or 6 months after childbirth. ........... 47 Analysis 1.3. Comparison 1 Nicotine replacement therapy versus control (Primary -

18 December 2020 – to Date)
(18 December 2020 – to date) MEDICINES AND RELATED SUBSTANCES ACT 101 OF 1965 (Gazette No. 1171, Notice No. 1002 dated 7 July 1965. Commencement date: 1 April 1966 [Proc. No. 94, Gazette No. 1413] SCHEDULES Government Notice 935 in Government Gazette 31387 dated 5 September 2008. Commencement date: 5 September 2008. As amended by: Government Notice R1230 in Government Gazette 32838 dated 31 December 2009. Commencement date: 31 December 2009. Government Notice R227 in Government Gazette 35149 dated 15 March 2012. Commencement date: 15 March 2012. Government Notice R674 in Government Gazette 36827 dated 13 September 2013. Commencement date: 13 September 2013. Government Notice R690 in Government Gazette 36850 dated 20 September 2013. Commencement date: 20 September 2013. Government Notice R104 in Government Gazette 37318 dated 11 February 2014. Commencement date: 11 February 2014. Government Notice R352 in Government Gazette 37622 dated 8 May 2014. Commencement date: 8 May 2014. Government Notice R234 in Government Gazette 38586 dated 20 March 2015. Commencement date: 20 March 2015. Government Notice 254 in Government Gazette 39815 dated 15 March 2016. Commencement date: 15 March 2016. Government Notice 620 in Government Gazette 40041 dated 3 June 2016. Commencement date: 3 June 2016. Prepared by: Page 2 of 199 Government Notice 748 in Government Gazette 41009 dated 28 July 2017. Commencement date: 28 July 2017. Government Notice 1261 in Government Gazette 41256 dated 17 November 2017. Commencement date: 17 November 2017. Government Notice R1098 in Government Gazette 41971 dated 12 October 2018. Commencement date: 12 October 2018. Government Notice R1262 in Government Gazette 42052 dated 23 November 2018. -

Substance Use in Pregnancy
Policy Clinical Guideline South Australian Perinatal Practices Guidelines – Substance use in Pregnancy Policy developed by: SA Maternal and Neonatal Clinical Network Approved SA Health Safety & Quality Strategic Governance Committee on: 8 October 2013 Next review due: 30 August 2016 Summary Guideline for the management of the pregnant woman with substance use. Keywords fetal, alcohol, tobacco, psychostimulants, opioids, midwifery, obstetric, contraception, unplanned pregnancies, blood-borne, viruses, substance, Perinatal Practices Guidelines, Substance use in Pregnancy, clinical guideline Policy history Is this a new policy? No Does this policy amend or update an existing policy? Yes Does this policy replace an existing policy? Yes If so, which policies? Substance use in Pregnancy Applies to All SA Health Portfolio All Department for Health and Ageing Divisions All Health Networks CALHN, SALHN, NALHN, CHSALHN, WCHN, SAAS Other Staff impact All Clinical, Medical, Nursing, Allied Health, Emergency, Dental, Mental Health, Pathology PDS reference CG117 Version control and change history Version Date from Date to Amendment 1.0 27 Jul 04 28 Oct 08 Original version 2.0 28 Oct 08 03 Mar 09 Breastfeeding reviewed 3.0 03 Mar 09 30 Nov 09 Labour and birth reviewed 4.0 14 Aug 13 Current NAS section removed © Department for Health and Ageing, Government of South Australia. All rights reserved. South Australian Perinatal Practice Guidelines substance use in pregnancy © Department of Health, Government of South Australia. All rights reserved. Note This guideline provides advice of a general nature. This statewide guideline has been prepared to promote and facilitate standardisation and consistency of practice, using a multidisciplinary approach. The guideline is based on a review of published evidence and expert opinion. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

00005721-201907000-00003.Pdf
2.0 ANCC Contact Hours Angela Y. Stanley, DNP, APRN-BC, PHCNS-BC, NEA-BC, RNC-OB, C-EFM, Catherine O. Durham, DNP, FNP-BC, James J. Sterrett, PharmD, BCPS, CDE, and Jerrol B. Wallace, DNP, MSN, CRNA SAFETY OF Over-the-Counter MEDICATIONS IN PREGNANCY Abstract Approximately 90% of pregnant women use medications while they are pregnant including both over-the-counter (OTC) and prescription medications. Some medica- tions can pose a threat to the pregnant woman and fetus with 10% of all birth defects directly linked to medications taken during pregnancy. Many medications have docu- mented safety for use during pregnancy, but research is limited due to ethical concerns of exposing the fetus to potential risks. Much of the information gleaned about safety in pregnancy is collected from registries, case studies and reports, animal studies, and outcomes management of pregnant women. Common OTC categories of readily accessible medications include antipyretics, analgesics, nonsteroidal anti- infl ammatory drugs, nasal topicals, antihistamines, decongestants, expectorants, antacids, antidiar- rheal, and topical dermatological medications. We review the safety categories for medications related to pregnancy and provide an overview of OTC medications a pregnant woman may consider for management of common conditions. Key words: Pharmacology; Pregnancy; Safety; Self-medication. Shutterstock 196 volume 44 | number 4 July/August 2019 Copyright © 2019 Wolters Kluwer Health, Inc. All rights reserved. he increased prevalence of pregnant women identifi ed risks in animal-reproduction studies or completed taking medications, including over-the-counter animal studies show no harm. The assignment of Category (OTC) medications presents a challenge to C has two indications; (1) limited or no research has been nurses providing care to women of childbear- conducted about use in pregnancy, and (2) animal studies ing age. -

Cardiovascular Medications in Pregnancy a Primer
Cardiovascular Medications in Pregnancy A Primer Karen L. Florio, DOa,b,*, Christopher DeZorzi, MDb,c, Emily Williams, MDb, Kathleen Swearingen, MSN, APRN, FNP-Ca, Anthony Magalski, MD, FHFSAb,c KEYWORDS Cardio-obstetrics Cardiac medications Pregnancy Medication safety Heart disease in pregnancy KEY POINTS Pregnancy induces dramatic physiologic and anatomic changes in the cardiovascular system beginning in the first trimester and continuing through the postpartum period. Maternal cardiac arrhythmias during pregnancy are a common occurrence and treatment should be considered from both obstetric and electrophysiologic standpoints. Anticoagulation and antiplatelet medications are prescribed commonly in both cardiology and ob- stetrics for various reasons. INTRODUCTION such, clinicians need to have a breadth of funda- mental knowledge regarding the safety profiles of Cardiovascular disease and its related disorders medication use during pregnancy. Many different are the leading causes of maternal morbidity and 1,2 factors need to be balanced when deciding on mortality in the United States. The increasing medications during pregnancy, including gesta- age at first pregnancy coupled with the rising rates tional age, reported teratogenicity, placental trans- of obesity likely contribute to this trend. More port, altered pharmacokinetics, route of women are desiring delayed fertility, which results administration, and dosing. Safety profiles are in higher rates of hypertensive disorders during 3 limited because reports of teratogenic effects are gestation. Increases in women with repaired from observational trials or registries due to the congenital cardiovascular disease becoming ethical dilemma of research on this vulnerable pregnant also add to this patient population. As a population.4 The purpose of this review is to guide result, the use of cardiovascular medications dur- clinical decisions for both obstetricians and cardi- ing pregnancy also is increasing. -

Hexoprenaline: Β-Adrenoreceptor Selectivity in Isolated Tissues from the Guinea-Pig
Clinical and Experimental Pharmacology and Physiology (1975) 2, 541-547. Hexoprenaline: p-adrenoreceptor selectivity in isolated tissues from the guinea-pig Stella R. O’Donnell and Janet C. Wanstall Department of Physiology, University of Queenstand, Brisbane, Australia (Received 12 March 1975; revision received 22 April 1975) SUMMARY 1. A catecholamine P-adrenoreceptor agonist, hexoprenaline, was examined in vitro on five guinea-pig tissues and its potency relative to isoprenaline (as 100) obtained. 2. Hexoprenaline clearly delineated between those tissues classified as containing P,-adrenoreceptors (trachea, hind limb blood vessels and uterus; relative potencies 219, 110 and 76 respectively) and those classified as containing P,-adrenoreceptors (atria and ileum; relative potencies 3.3 and 1.0 respectively). 3. Hexoprenaline differed from some previously studied noncatecholamine P-adrenoreceptor agonists in being only two-fold less potent, relative to isoprena- line, as a vasodilator in perfused hind limb than as a tracheal relaxant. Key words : j3-adrenoreceptors, blood vessels, bronchodilators, guinea-pig, hexo- prenaline, selectivity, trachea. INTRODUCTION In a previous study using in vitro preparations from the guinea-pig, O’Donnell & Wanstall (1974a) examined potential sympathomimetic bronchodilator compounds for their potency as tracheal relaxants (p,-adrenoreceptors), atrial stimulants (P,-adrenoreceptors) and vasodilators in the perfused hind limb (p2-adrenoreceptors). Some resorcinolamines showed selectivity for trachea compared with not only atria but also blood vessels. There is some evidence in dogs in vivo that other noncatecholamines, namely carbuterol (Wardell et al., 1974), and salbutamol and terbutaline (Wasserman & Levy, 1974), display similar select- ivity. If selectivity between respiratory and vascular smooth muscle represents selectivity at the p-adrenoreceptor level, it poses the question whether the P1/p2subclassification (Lands et al., 1967a; Lands, Luduena & Buzzo, 1967b) is too rigid. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -
100 Storage Condition=50 C/Ambrh
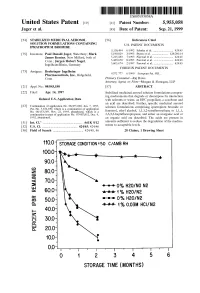
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

Ethanol and Tobacco Abuse in Pregnancy: Anaesthetic Considerations
REVIEW ARTICLE Ethanol and tobacco abuse in pregnancy: Anaesthetic considerations K M Kuczkowski M.D, Assistant Clinical Professor of Anesthesiology and Reproductive Medicine, Co-Director of Obstetric Anesthesia, Departments of Anesthesiology and Reproductive Medicine, University of California, San Diego , USA Introduction The illicit drug abuse in pregnancy has received significant attention over the past two decades.1 However, far too little attention has been given to the consequences of the use of social drugs such as ethanol and tobacco, which are by far the most commonly abused substances during pregnancy. While the deleterious effects of cocaine or amphetamines on the mother and the fetus are more pronounced and easier to detect, the addiction to ethanol and tobacco is usually subtle and more difficult to diagnose.1, 2-4 As a result recreational use of alcohol and tobacco may continue undetected in pregnancy, significantly effecting pregnancy outcome and obstetric and anaesthetic management of these patients. This article reviews the consequences of ethanol and tobacco use in pregnancy and offers recommendation for anaesthetic management of these potentially complicated pregnancies. General considerations rette smoking.2, 17 The American College of Obstetricians and Substance abuse is defined as “self-administration of various drugs Gynaecologists (ACOG) has made multiple recommendations regard- that deviates from medically or socially accepted use, which if pro- ing management of patients with drug abuse during pregnancy. longed can lead to the development of physical and psychological Women who acknowledge use of illicit substance during pregnancy dependence”.5 This chemical dependency is characterized by peri- should be counseled and offered necessary treatment. -

Cardiovascular Disasters in Pregnancy
Cardiovascular Disasters in Pregnancy Sarah K. Sommerkamp, MD, RDMS*, Alisa Gibson, MD, DMD KEYWORDS Pregnancy Pulmonary embolus Aortic dissection Cardiomyopathy Acute myocardial infarction Arrhythmia Cardiac arrest Perimortem cesarean section KEY POINTS During pregnancy, the D-dimer level is likely to be elevated; however, a negative D-dimer is still reliably negative in a patient with low pretest probability. Life-threatening pulmonary embolism can be treated with tissue plasminogen activator, despite the relative contraindication of pregnancy. Aortic dissection is more common in pregnancy and in the immediate postpartum period than their nonpregnant counterparts. Cardiac disease in pregnancy is becoming more common, because of the increasing incidence of advanced maternal age, obesity, hypertension, and diabetes mellitus and improved treatments for congenital heart disease. Percutaneous coronary intervention is first-line therapy for pregnant patients with acute myocardial infarction. Displacement of the uterus is imperative to a successful resuscitation. Drug and electricity doses are unchanged in resuscitation of the pregnant patient. Perimortem cesarean section must be completed within 5 minutes of loss of circulation. INTRODUCTION The normal changes that occur during pregnancy are demanding on the cardiovas- cular system. Total cardiac output increases about 50% from a combination of in- creased blood volume and pulse along with a decrease in peripheral resistance. These changes can place significant stress on a normal heart. Physiologic changes are even more dangerous to individuals with underlying cardiac disorders. Cardiac arrest can occur in previously asymptomatic women who are experiencing this type of cardiac stress. In its 2010 guidelines,1 the American Heart Association summarized Funding sources: None. Conflict of interest: None.