Hexoprenaline: Β-Adrenoreceptor Selectivity in Isolated Tissues from the Guinea-Pig
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

18 December 2020 – to Date)
(18 December 2020 – to date) MEDICINES AND RELATED SUBSTANCES ACT 101 OF 1965 (Gazette No. 1171, Notice No. 1002 dated 7 July 1965. Commencement date: 1 April 1966 [Proc. No. 94, Gazette No. 1413] SCHEDULES Government Notice 935 in Government Gazette 31387 dated 5 September 2008. Commencement date: 5 September 2008. As amended by: Government Notice R1230 in Government Gazette 32838 dated 31 December 2009. Commencement date: 31 December 2009. Government Notice R227 in Government Gazette 35149 dated 15 March 2012. Commencement date: 15 March 2012. Government Notice R674 in Government Gazette 36827 dated 13 September 2013. Commencement date: 13 September 2013. Government Notice R690 in Government Gazette 36850 dated 20 September 2013. Commencement date: 20 September 2013. Government Notice R104 in Government Gazette 37318 dated 11 February 2014. Commencement date: 11 February 2014. Government Notice R352 in Government Gazette 37622 dated 8 May 2014. Commencement date: 8 May 2014. Government Notice R234 in Government Gazette 38586 dated 20 March 2015. Commencement date: 20 March 2015. Government Notice 254 in Government Gazette 39815 dated 15 March 2016. Commencement date: 15 March 2016. Government Notice 620 in Government Gazette 40041 dated 3 June 2016. Commencement date: 3 June 2016. Prepared by: Page 2 of 199 Government Notice 748 in Government Gazette 41009 dated 28 July 2017. Commencement date: 28 July 2017. Government Notice 1261 in Government Gazette 41256 dated 17 November 2017. Commencement date: 17 November 2017. Government Notice R1098 in Government Gazette 41971 dated 12 October 2018. Commencement date: 12 October 2018. Government Notice R1262 in Government Gazette 42052 dated 23 November 2018. -

The Effects of Adrenergic Amines and Theophylline On
THE EFFECTS OF ADRENERGIC AMINES AND THEOPHYLLINE ON CONTRACTILE FORCE AND CYCLIC AMP by Terry T. Martinez M.S., Purdue University, U.S.A. 1970 B.S., Purdue University, U.S.A. 1969 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in the Division of Pharmacology of the Faculty of Pharmaceutical Sciences We accept this thesis as conforming to the required standard. THE UNIVERSITY OF BRITISH COLUMBIA October, 1975 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of Pharmaceutical Sciences The University of British Columbia 2075 Wesbrook Place Vancouver, Canada V6T 1W5 Date October 14, 1975 ABSTRACT Time response studies of the effects of norepinephrine and phenylephrine revealed that both agonists caused an increase in cyclic AMP levels prior to increases in contractile force. Norepinephrine caused a nearly six fold increase in cyclic AMP, whereas phenylephrine produced only a 50% increase in the nucleo• tide. Pretreatment with reserpine did not affect the norepineph• rine cyclic AMP response; however, the phenylephrine cyclic AMP response was abolished. Reserpine pretreatment did not significant• ly affect the contractile responses of either amine. -

Transdermal Drug Delivery Device Including An
(19) TZZ_ZZ¥¥_T (11) EP 1 807 033 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61F 13/02 (2006.01) A61L 15/16 (2006.01) 20.07.2016 Bulletin 2016/29 (86) International application number: (21) Application number: 05815555.7 PCT/US2005/035806 (22) Date of filing: 07.10.2005 (87) International publication number: WO 2006/044206 (27.04.2006 Gazette 2006/17) (54) TRANSDERMAL DRUG DELIVERY DEVICE INCLUDING AN OCCLUSIVE BACKING VORRICHTUNG ZUR TRANSDERMALEN VERABREICHUNG VON ARZNEIMITTELN EINSCHLIESSLICH EINER VERSTOPFUNGSSICHERUNG DISPOSITIF D’ADMINISTRATION TRANSDERMIQUE DE MEDICAMENTS AVEC COUCHE SUPPORT OCCLUSIVE (84) Designated Contracting States: • MANTELLE, Juan AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Miami, FL 33186 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • NGUYEN, Viet SK TR Miami, FL 33176 (US) (30) Priority: 08.10.2004 US 616861 P (74) Representative: Awapatent AB P.O. Box 5117 (43) Date of publication of application: 200 71 Malmö (SE) 18.07.2007 Bulletin 2007/29 (56) References cited: (73) Proprietor: NOVEN PHARMACEUTICALS, INC. WO-A-02/36103 WO-A-97/23205 Miami, FL 33186 (US) WO-A-2005/046600 WO-A-2006/028863 US-A- 4 994 278 US-A- 4 994 278 (72) Inventors: US-A- 5 246 705 US-A- 5 474 783 • KANIOS, David US-A- 5 474 783 US-A1- 2001 051 180 Miami, FL 33196 (US) US-A1- 2002 128 345 US-A1- 2006 034 905 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research
Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Garabedian, Laura Faden. 2013. Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:11156786 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research A dissertation presented by Laura Faden Garabedian to The Committee on Higher Degrees in Health Policy in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Health Policy Harvard University Cambridge, Massachusetts March 2013 © 2013 – Laura Faden Garabedian All rights reserved. Professor Stephen Soumerai Laura Faden Garabedian Quasi-Experimental Health Policy Research: Evaluation of Universal Health Insurance and Methods for Comparative Effectiveness Research Abstract This dissertation consists of two empirical papers and one methods paper. The first two papers use quasi-experimental methods to evaluate the impact of universal health insurance reform in Massachusetts (MA) and Thailand and the third paper evaluates the validity of a quasi- experimental method used in comparative effectiveness research (CER). My first paper uses interrupted time series with data from IMS Health to evaluate the impact of Thailand’s universal health insurance and physician payment reform on utilization of medicines for three non-communicable diseases: cancer, cardiovascular disease and diabetes. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Toxic, and Comatose-Fatal Blood-Plasma Concentrations (Mg/L) in Man
Therapeutic (“normal”), toxic, and comatose-fatal blood-plasma concentrations (mg/L) in man Substance Blood-plasma concentration (mg/L) t½ (h) Ref. therapeutic (“normal”) toxic (from) comatose-fatal (from) Abacavir (ABC) 0.9-3.9308 appr. 1.5 [1,2] Acamprosate appr. 0.25-0.7231 1311 13-20232 [3], [4], [5] Acebutolol1 0.2-2 (0.5-1.26)1 15-20 3-11 [6], [7], [8] Acecainide see (N-Acetyl-) Procainamide Acecarbromal(um) 10-20 (sum) 25-30 Acemetacin see Indomet(h)acin Acenocoumarol 0.03-0.1197 0.1-0.15 3-11 [9], [3], [10], [11] Acetaldehyde 0-30 100-125 [10], [11] Acetaminophen see Paracetamol Acetazolamide (4-) 10-20267 25-30 2-6 (-13) [3], [12], [13], [14], [11] Acetohexamide 20-70 500 1.3 [15] Acetone (2-) 5-20 100-400; 20008 550 (6-)8-31 [11], [16], [17] Acetonitrile 0.77 32 [11] Acetyldigoxin 0.0005-0.00083 0.0025-0.003 0.005 40-70 [18], [19], [20], [21], [22], [23], [24], [25], [26], [27] 1 Substance Blood-plasma concentration (mg/L) t½ (h) Ref. therapeutic (“normal”) toxic (from) comatose-fatal (from) Acetylsalicylic acid (ASS, ASA) 20-2002 300-3502 (400-) 5002 3-202; 37 [28], [29], [30], [31], [32], [33], [34] Acitretin appr. 0.01-0.05112 2-46 [35], [36] Acrivastine -0.07 1-2 [8] Acyclovir 0.4-1.5203 2-583 [37], [3], [38], [39], [10] Adalimumab (TNF-antibody) appr. 5-9 146 [40] Adipiodone(-meglumine) 850-1200 0.5 [41] Äthanol see Ethanol -139 Agomelatine 0.007-0.3310 0.6311 1-2 [4] Ajmaline (0.1-) 0.53-2.21 (?) 5.58 1.3-1.6, 5-6 [3], [42] Albendazole 0.5-1.592 8-992 [43], [44], [45], [46] Albuterol see Salbutamol Alcuronium 0.3-3353 3.3±1.3 [47] Aldrin -0.0015 0.0035 50-1676 (as dieldrin) [11], [48] Alendronate (Alendronic acid) < 0.005322 -6 [49], [50], [51] Alfentanil 0.03-0.64 0.6-2.396 [52], [53], [54], [55] Alfuzosine 0.003-0.06 3-9 [8] 2 Substance Blood-plasma concentration (mg/L) t½ (h) Ref. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -
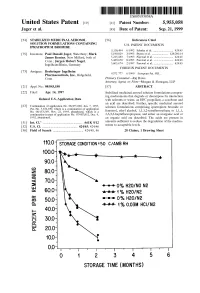
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

Addition of Intravenous Beta2-Agonists to Inhaled Beta2- Agonists for Acute Asthma (Review)
Addition of intravenous beta2-agonists to inhaled beta2- agonists for acute asthma (Review) Travers AH, Milan SJ, Jones AP, Camargo Jr CA, Rowe BH This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2012, Issue 12 http://www.thecochranelibrary.com Addition of intravenous beta2-agonists to inhaled beta2-agonists for acute asthma (Review) Copyright © 2012 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 3 BACKGROUND .................................... 5 OBJECTIVES ..................................... 5 METHODS ...................................... 5 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 12 AUTHORS’CONCLUSIONS . 12 ACKNOWLEDGEMENTS . 13 REFERENCES ..................................... 13 CHARACTERISTICSOFSTUDIES . 19 DATAANDANALYSES. 31 Analysis 1.1. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 1 Admissions. 31 Analysis 1.2. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 2 Length of stay. 32 Analysis 1.3. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 3 Pulse rate at 2 -

Quick Screening of Priority Beta-Agonists in Urine Using Automated Turboflow™ - LC/Exactive Mass Spectrometry Thorsten Bernsmann, Peter Fuerst, Michal Godula
Quick screening of priority beta-agonists in urine using automated TurboFlow™ - LC/Exactive mass spectrometry Thorsten Bernsmann, Peter Fuerst, Michal Godula To cite this version: Thorsten Bernsmann, Peter Fuerst, Michal Godula. Quick screening of priority beta-agonists in urine using automated TurboFlow™ - LC/Exactive mass spectrometry. Food Additives and Contaminants, 2011, 28 (10), pp.1352-1363. 10.1080/19440049.2011.619504. hal-00743048 HAL Id: hal-00743048 https://hal.archives-ouvertes.fr/hal-00743048 Submitted on 18 Oct 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Food Additives and Contaminants This paper describes a method for the determination of priority β -agonists in urine based on a fully automated sample preparation procedure using the online TurboFlow™ chromatography clean -up step and determination on the Orbitrap™ mass analyzer technology. The principle of the method after enzymatic hydrolysis over night on a small column packed with a special stationary phase (TurboFlow™) while flushing away sample matrix and interfering compounds. Thereafter the analytes are transferred onto an analytical column and detected by id chromatography/high resolution mass spectrometry in full scan mode at a resolution of R=50,000 FWHM (full width at half maximum) and in HCD (Higher Energy Collisional Dissociation) scan mode at a resolving power of 10,000 FWHM. -

210428Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 210428Orig1s000 OTHER REVIEW(S) DIVISION OF CARDIOVASCULAR AND RENAL PRODUCTS Regulatory Project Manager Overview I. GENERAL INFORMATION NDA: 210428 Drug: Metoprolol Succinate Extended-Release Capsules, 25 mg, 50 mg, 100 mg and 200 mg Class: Beta- Blocker Applicant: Sun Pharmaceutical Industries Limited Proposed Indications: Hypertension, Angina Pectoris & Heart Failure Date of submission: March 30, 2017 PDUFA date: January 30, 2018 II. REVIEW TEAM Office of New Drugs, Office of Drug Evaluation I: Division of Cardiovascular & Renal Product Norman Stockbridge, MD, PhD, Director Mary Ross Southworth, PharmD, Deputy Director for Safety Michael Monteleone, MS, RAC, Assistant Director for Labeling Martina Sahre, PhD, Cross Discipline Team Leader Fortunato Senatore, MD, Clinical Reviewer Albert DeFelice, PhD, Non-Clinical Supervisor Muriel Saulnier, PhD, Non-Clinical Reviewer Edward Fromm, RPh, RAC, Chief, Project Management Staff Maryam Changi, PharmD, Regulatory Project Manager Office of Pharmaceutical Quality: Wendy Wilson-Lee, PhD, Application Technical Lead Grafton Adams, Regulatory Business Process Manager Milton Sloan, PhD, Drug Product Reviewer Rohit Tiwari, PhD, Drug Substance Reviewer Ben Stevens, PhD, Drug Substance Team Leader Kaushal Dave, PhD, Biopharmaceutical Reviewer Wu, Ta-Chin, PhD, Biopharmaceutical Team Leader Viviana Matta, PhD, Facility Reviewer Ruth Moore, PhD, Facility Team Leader Chunsheng Cai, PhD, Process Reviewer NDA 210428 RPM review Page 1 Reference ID: 42132034213643 Labeling/PMC-PMR to Sponsor: November 30, 2017 PDUFA Date: January 30, 2018 (Standard, 10-Month) PDUFA Goal Date: January 30, 2018 Approval letter: January 26, 2018 7. Reviews a) Divisional Memorandum: (January 26, 2018) Dr. Stockbridge indicated his concurrence on Dr. Sahre’s CDTL memo.