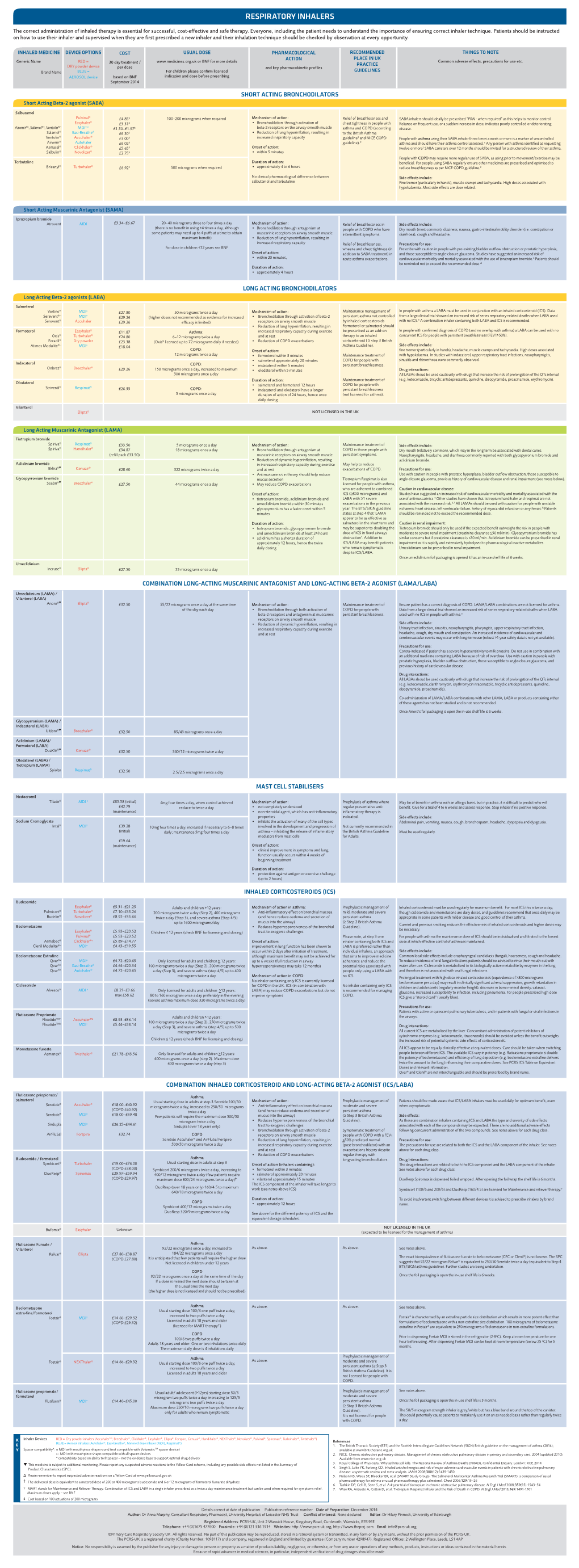
Respiratory Inhalers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Effect of Indacaterol, a Novel Long-Acting Beta 2
ERJ Express. Published on November 29, 2006 as doi: 10.1183/09031936.00032806 EFFECT OF INDACATEROL, A NOVEL LONG-ACTING BETA 2-AGONIST, ON HUMAN ISOLATED BRONCHI Emmanuel Naline (1), Alexandre Trifilieff (2), Robin A. Fairhurst (2), Charles Advenier (1), Mathieu Molimard (3) (1) Research Unit EA220, Université de Versailles, Faculté de Médecine, Pharmacology, Hôpital Foch, 40 rue Worth, 92150 Suresnes, France (2) Novartis Institute for BioMedical Research, Horsham, England (3) Département de Pharmacologie, Université Victor Segalen Bordeaux 2; CHU Pellegrin; INSERM U657, Bordeaux, France Correspondence: Mathieu Molimard Département de Pharmacologie CHU de Bordeaux - Université Victor Segalen-INSERM U657 33076 Bordeaux cedex, France Phone: 33 (0)5 57 57 15 60 Fax: 33 (0)5 57 57 46 71 E-mail: [email protected] Short title: Indacaterol effect on isolated human bronchi 1 Copyright 2006 by the European Respiratory Society. Abstract Indacaterol is a novel β2-adrenoceptor agonist in development for the once-daily treatment of asthma and COPD. This study evaluated the relaxant effect of indacaterol on isolated human bronchi obtained from lungs of patients undergoing surgery for lung carcinoma. Potency (-logEC50), intrinsic efficacy (Emax) and onset of action were determined at resting tone. Duration of action was determined against cholinergic neural contraction induced by electrical field stimulation (EFS). At resting tone, -logEC50 and Emax values were, respectively, 8.82±0.41 and 77±5% for indacaterol, 9.84±0.22 and 94±1% for formoterol, 8.36±0.16 and 74±4% for salmeterol, and 8.43±0.22 and 84±4% for salbutamol. In contrast to salmeterol, indacaterol did not antagonize the isoprenaline response. -

TAYSIDE PRESCRIBER Issue No
TAYSIDE PRESCRIBER Issue No. 122 – May 2012 Produced by the NS Tayside Medicines Governance Unit in conjunction with Mental Health Citalopram & escitalopram:QT interval prolongation New maximum daily dose restrictions, contraindications, and warnings Information has been issued via Drug Safety Update, Volume 5, Issue 5, December 2011 and ‘Dear Healthcare Professional Letters’ for both citalopram and escitalopram regarding new restrictions on the maximum daily doses, contraindications, and warnings. This is as a result of an assessment of a QT study that revealed dose-dependent increase in the QT interval observed with ECG monitoring for both citalopram and escitalopram. Maximum licensed daily doses for citalopram and escitalopram Adults Adults > 65 years Adults with hepatic impairment Citalopram 40 mg 20 mg 20 mg Escitalopram (non-formulary) 20 mg 10 mg 10mg The guidance in NHS Tayside is: ⇒ to review all patients on high dose* citalopram or escitalopram with aim of reducing to new maximum licensed doses ( * above new maximum licensed daily doses as stated in the table above) ⇒ not to prescribe citalopram or escitalopram with other medication known to prolong the QT interval ⇒ not to prescribe citalopram and escitalopram in patients with known QT prolongation or congenital long QT syndrome ⇒ to consider alternative antidepressant in patients with cardiac disease ( e.g. patients with significant bradycardia; recent myocardial infarction or decompensated heart failure) See flow diagram on page 3 for further guidance and table below on medicines known to prolong the QT interval. Medicines known to increase plasma levels of citalopram or escitalopram, e.g. omeprazole & some antivirals may require dose reduction of citalopram or escitalopram and should be used with caution. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

PRODUCT MONOGRAPH ELAVIL® Amitriptyline Hydrochloride Tablets
PRODUCT MONOGRAPH ELAVIL® amitriptyline hydrochloride tablets USP 10, 25, 50 and 75 mg Antidepressant AA PHARMA INC. DATE OF PREPARATION: 1165 Creditstone Road Unit #1 August 29, 2018 Vaughan, ON L4K 4N7 Control No.: 217626 1 PRODUCT MONOGRAPH ELAVIL® amitriptyline hydrochloride tablets USP 10, 25, 50, 75 mg THERAPEUTIC CLASSIFICATION Antidepressant ACTIONS AND CLINICAL PHARMACOLOGY Amitriptyline hydrochloride is a tricyclic antidepressant with sedative properties. Its mechanism of action in man is not known. Amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain. Amitriptyline has pronounced anticholinergic properties and produces EKG changes and quinidine-like effects on the heart (See ADVERSE REACTIONS). It also lowers the convulsive threshold and causes alterations in EEG and sleep patterns. Orally administered amitriptyline is readily absorbed and rapidly metabolized. Steady-state plasma concentrations vary widely and this variation may be genetically determined. Amitriptyline is primarily excreted in the urine, mostly in the form of metabolites, with some excretion also occurring in the feces. INDICATIONS AND CLINICAL USE ELAVIL® (amitriptyline hydrochloride) is indicated in the drug management of depressive illness. ELAVIL® may be used in depressive illness of psychotic or endogenous nature and in selected patients with neurotic depression. Endogenous depression is more likely to be alleviated than are other depressive states. ELAVIL® ®, because of its sedative action, is also of value in alleviating the anxiety component of depression. As with other tricyclic antidepressants, ELAVIL® may precipitate hypomanic episodes in patients with bipolar depression. These drugs are not indicated in mild depressive states and depressive reactions. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

PERPHENAZINE and AMITRIPTYLINE HYDROCHLORIDE- Perphenazine and Amitriptyline Hydrochloride Tablet, Film Coated Mylan Pharmaceuticals Inc
PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE- perphenazine and amitriptyline hydrochloride tablet, film coated Mylan Pharmaceuticals Inc. ---------- WARNING Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug- treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine and amitriptyline hydrochloride is not approved for the treatment of patients with dementia- related psychosis (see WARNINGS). Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of perphenazine and amitriptyline or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. -

Nuedexta) National Drug Monograph May 2013 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives
Dextromethorphan/ Quinidine Monograph Dextromethorphan Hydrobromide and Quinidine Sulfate (Nuedexta) National Drug Monograph May 2013 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. These documents will be updated when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. Executive Summary: Current therapy for pseudobulbar affect (PBA) typically utilizes non-pharmacologic coping strategies such as relaxation and distraction techniques. The off-label use of selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and dopaminergic agents has found limited success in treating PBA exacerbations. Dextromethorphan/quinidine (Nuedexta) is a combination of two existing generic formulary options; however quinidine is not available in an appropriate strength or in a liquid formulation (200mg, 300mg capsules only). Low dose quinidine inhibits the rapid metabolism of dextromethorphan, therefore increasing the bioavailability of dextromethorphan, resulting in effective plasma levels. In a double-blind, placebo-controlled study, the combination of dextromethorphan (20 or 30 mg) and low dose quinidine (10 mg) [STAR trial] significantly reduced the frequency of pseudobulbar affect episodes vs. placebo over the course of 12 weeks of treatment (p < 0.0001). Patients in the dextromethorphan/quinidine 20 mg/10 mg group reported a mean weekly episode reduction of 82% from baseline vs. a reduction of 47% in the placebo group (p = 0.001). Mean Center for Neurological Study-Lability Scale (CNS-LS) scores decreased by 8.2 points for both dextromethorphan/quinidine dosage groups vs. -

Analysis of the Indacaterol-Regulated Transcriptome in Human Airway
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2018/04/13/jpet.118.249292.DC1 1521-0103/366/1/220–236$35.00 https://doi.org/10.1124/jpet.118.249292 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 366:220–236, July 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Analysis of the Indacaterol-Regulated Transcriptome in Human Airway Epithelial Cells Implicates Gene Expression Changes in the s Adverse and Therapeutic Effects of b2-Adrenoceptor Agonists Dong Yan, Omar Hamed, Taruna Joshi,1 Mahmoud M. Mostafa, Kyla C. Jamieson, Radhika Joshi, Robert Newton, and Mark A. Giembycz Departments of Physiology and Pharmacology (D.Y., O.H., T.J., K.C.J., R.J., M.A.G.) and Cell Biology and Anatomy (M.M.M., R.N.), Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada Received March 22, 2018; accepted April 11, 2018 Downloaded from ABSTRACT The contribution of gene expression changes to the adverse and activity, and positive regulation of neutrophil chemotaxis. The therapeutic effects of b2-adrenoceptor agonists in asthma was general enriched GO term extracellular space was also associ- investigated using human airway epithelial cells as a therapeu- ated with indacaterol-induced genes, and many of those, in- tically relevant target. Operational model-fitting established that cluding CRISPLD2, DMBT1, GAS1, and SOCS3, have putative jpet.aspetjournals.org the long-acting b2-adrenoceptor agonists (LABA) indacaterol, anti-inflammatory, antibacterial, and/or antiviral activity. Numer- salmeterol, formoterol, and picumeterol were full agonists on ous indacaterol-regulated genes were also induced or repressed BEAS-2B cells transfected with a cAMP-response element in BEAS-2B cells and human primary bronchial epithelial cells by reporter but differed in efficacy (indacaterol $ formoterol . -

Trimbow, INN-Beclometasone Dipropionate, Formoterol Fumarate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Trimbow 87 micrograms/5 micrograms/9 micrograms pressurised inhalation, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece) contains 87 micrograms of beclometasone dipropionate, 5 micrograms of formoterol fumarate dihydrate and 9 micrograms of glycopyrronium (as 11 micrograms glycopyrronium bromide). Each metered dose (the dose leaving the valve) contains 100 micrograms of beclometasone dipropionate, 6 micrograms of formoterol fumarate dihydrate and 10 micrograms of glycopyrronium (as 12.5 micrograms glycopyrronium bromide). Excipient with known effect Trimbow contains 8.856 mg ethanol per actuation. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Pressurised inhalation, solution (pressurised inhalation). Colourless to yellowish liquid solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications COPD Maintenance treatment in adult patients with moderate to severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an inhaled corticosteroid and a long-acting beta2-agonist or a combination of a long-acting beta2-agonist and a long-acting muscarinic antagonist (for effects on symptoms control and prevention of exacerbations see section 5.1). Asthma Maintenance treatment of asthma, in adults not adequately controlled with a maintenance combination of a long-acting beta2-agonist and medium dose of inhaled corticosteroid, and who experienced one or more asthma exacerbations in the previous year. 4.2 Posology and method of administration Posology Adults The recommended dose is two inhalations twice daily. The maximum dose is two inhalations twice daily. Patients should be advised to take Trimbow every day even when asymptomatic. -

ANTIDEPRESSANTS in USE in CLINICAL PRACTICE Mark Agius1 & Hannah Bonnici2 1Clare College, University of Cambridge, Cambridge, UK 2Hospital Pharmacy St
Psychiatria Danubina, 2017; Vol. 29, Suppl. 3, pp 667-671 Conference paper © Medicinska naklada - Zagreb, Croatia ANTIDEPRESSANTS IN USE IN CLINICAL PRACTICE Mark Agius1 & Hannah Bonnici2 1Clare College, University of Cambridge, Cambridge, UK 2Hospital Pharmacy St. James Hospital Malta, Malta SUMMARY The object of this paper, rather than producing new information, is to produce a useful vademecum for doctors prescribing antidepressants, with the information useful for their being prescribed. Antidepressants need to be seen as part of a package of treatment for the patient with depression which also includes psychological treatments and social interventions. Here the main Antidepressant groups, including the Selective Serotonin uptake inhibiters, the tricyclics and other classes are described, together with their mode of action, side effects, dosages. Usually antidepressants should be prescribed for six months to treat a patient with depression. The efficacy of anti-depressants is similar between classes, despite their different mechanisms of action. The choice is therefore based on the side-effects to be avoided. There is no one ideal drug capable of exerting its therapeutic effects without any adverse effects. Increasing knowledge of what exactly causes depression will enable researchers not only to create more effective antidepressants rationally but also to understand the limitations of existing drugs. Key words: antidepressants – depression - psychological therapies - social therapies * * * * * Introduction Monoamine oxidase (MAO) inhibitors í Non-selective Monoamine Oxidase Inhibitors Depression may be defined as a mood disorder that í Selective Monoamine Oxidase Type A inhibitors negatively and persistently affects the way a person feels, thinks and acts. Common signs include low mood, Atypical Anti-Depressants and other classes changes in appetite and sleep patterns and loss of inte- rest in activities that were once enjoyable.