Evidence of Atypical Fl-Adrenoceptors in Rat Colon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of the Serotonergic System and the Effects of Antidepressants During Brain Development Examined Using in Vivo Pet Imaging and in Vitro Receptor Binding
From THE DEPARTMENT OF CLINICAL NEUROSCIENCE Karolinska Institutet, Stockholm, Sweden THE ROLE OF THE SEROTONERGIC SYSTEM AND THE EFFECTS OF ANTIDEPRESSANTS DURING BRAIN DEVELOPMENT EXAMINED USING IN VIVO PET IMAGING AND IN VITRO RECEPTOR BINDING Stal Saurav Shrestha Stockholm 2014 Cover Illustration: Voxel-wise analysis of the whole monkey brain using the PET radioligand, [11C]DASB showing persistent serotonin transporter upregulation even after more than 1.5 years of fluoxetine discontinuation. All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Universitetsservice-AB © Stal Saurav Shrestha, 2014 ISBN 978-91-7549-522-4 Serotonergic System and Antidepressants During Brain Development To my family Amaze yourself ! Stal Saurav Shrestha, 2014 The Department of Clinical Neuroscience The role of the serotonergic system and the effects of antidepressants during brain development examined using in vivo PET imaging and in vitro receptor binding AKADEMISK AVHANDLING som för avläggande av medicine doktorsexamen vid Karolinska Institutet offentligen försvaras i CMM föreläsningssalen L8:00, Karolinska Universitetssjukhuset, Solna THESIS FOR DOCTORAL DEGREE (PhD) Stal Saurav Shrestha Date: March 31, 2014 (Monday); Time: 10 AM Venue: Center for Molecular Medicine Lecture Hall Floor 1, Karolinska Hospital, Solna Principal Supervisor: Opponent: Robert B. Innis, MD, PhD Klaus-Peter Lesch, MD, PhD National Institutes of Health University of Würzburg Department of NIMH Department -

The 2.1 A˚Resolution Structure of Cyanopindolol-Bound B1
The 2.1 A˚ Resolution Structure of Cyanopindolol-Bound + b1-Adrenoceptor Identifies an Intramembrane Na Ion that Stabilises the Ligand-Free Receptor Jennifer L. Miller-Gallacher.¤a, Rony Nehme´ ., Tony Warne, Patricia C. Edwards, Gebhard F. X. Schertler¤b¤c, Andrew G. W. Leslie, Christopher G. Tate* Structural Studies Division, MRC Laboratory of Molecular Biology, Cambridge, Cambridgeshire, United Kingdom Abstract The b1-adrenoceptor (b1AR) is a G protein-coupled receptor (GPCR) that is activated by the endogenous agonists adrenaline and noradrenaline. We have determined the structure of an ultra-thermostable b1AR mutant bound to the weak partial agonist cyanopindolol to 2.1 A˚ resolution. High-quality crystals (100 mm plates) were grown in lipidic cubic phase without the assistance of a T4 lysozyme or BRIL fusion in cytoplasmic loop 3, which is commonly employed for GPCR crystallisation. An intramembrane Na+ ion was identified co-ordinated to Asp872.50, Ser1283.39 and 3 water molecules, which is part of a more extensive network of water molecules in a cavity formed between transmembrane helices 1, 2, 3, 6 and 7. Remarkably, + this water network and Na ion is highly conserved between b1AR and the adenosine A2A receptor (rmsd of 0.3 A˚), despite an overall rmsd of 2.4 A˚ for all Ca atoms and only 23% amino acid identity in the transmembrane regions. The affinity of + agonist binding and nanobody Nb80 binding to b1AR is unaffected by Na ions, but the stability of the receptor is decreased by 7.5uC in the absence of Na+. Mutation of amino acid side chains that are involved in the co-ordination of either + Na or water molecules in the network decreases the stability of b1AR by 5–10uC. -

Adrenergic G-Protein- Coupled Receptor
Vol 454 | 24 July 2008 | doi:10.1038/nature07101 ARTICLES Structure of a b1-adrenergic G-protein- coupled receptor Tony Warne1, Maria J. Serrano-Vega1, Jillian G. Baker2, Rouslan Moukhametzianov1, Patricia C. Edwards1, Richard Henderson1, Andrew G. W. Leslie1, Christopher G. Tate1 & Gebhard F. X. Schertler1 G-protein-coupled receptors have a major role in transmembrane signalling in most eukaryotes and many are important drug targets. Here we report the 2.7 A˚ resolution crystal structure of a b1-adrenergic receptor in complex with the high-affinity antagonist cyanopindolol. The modified turkey (Meleagris gallopavo) receptor was selected to be in its antagonist conformation and its thermostability improved by earlier limited mutagenesis. The ligand-binding pocket comprises 15 side chains from amino acid residues in 4 transmembrane a-helices and extracellular loop 2. This loop defines the entrance of the ligand-binding pocket and is stabilized by two disulphide bonds and a sodium ion. Binding of cyanopindolol to the b1-adrenergic receptor and binding of carazolol to the b2-adrenergic receptor involve similar interactions. A short well-defined helix in cytoplasmic loop 2, not observed in either rhodopsin or the b2-adrenergic receptor, directly interacts by means of a tyrosine with the highly conserved DRY motif at the end of helix 3 that is essential for receptor activation. G-protein-coupled receptors (GPCRs) are a large family of integral These structures, both containing the high affinity antagonist cara- membrane proteins that are prevalent in eukaryotes from yeast to zolol, defined the overall architecture of b2AR and the structure of the man, and function as key intermediaries in the transduction of sig- ligand-binding pocket. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -

Drugs for Primary Prevention of Atherosclerotic Cardiovascular Disease: an Overview of Systematic Reviews
Supplementary Online Content Karmali KN, Lloyd-Jones DM, Berendsen MA, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol. Published online April 27, 2016. doi:10.1001/jamacardio.2016.0218. eAppendix 1. Search Documentation Details eAppendix 2. Background, Methods, and Results of Systematic Review of Combination Drug Therapy to Evaluate for Potential Interaction of Effects eAppendix 3. PRISMA Flow Charts for Each Drug Class and Detailed Systematic Review Characteristics and Summary of Included Systematic Reviews and Meta-analyses eAppendix 4. List of Excluded Studies and Reasons for Exclusion This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 eAppendix 1. Search Documentation Details. Database Organizing body Purpose Pros Cons Cochrane Cochrane Library in Database of all available -Curated by the Cochrane -Content is limited to Database of the United Kingdom systematic reviews and Collaboration reviews completed Systematic (UK) protocols published by by the Cochrane Reviews the Cochrane -Only systematic reviews Collaboration Collaboration and systematic review protocols Database of National Health Collection of structured -Curated by Centre for -Only provides Abstracts of Services (NHS) abstracts and Reviews and Dissemination structured abstracts Reviews of Centre for Reviews bibliographic -

(±)-Pindolol Acts As a Partial Agonist at Atypical >-Adrenoceptors in The
Jpn. J. Pharmacol. 85, 35 – 40 (2001) (±)-Pindolol Acts as a Partial Agonist at Atypical >-Adrenoceptors in the Guinea Pig Duodenum Takahiro Horinouchi and Katsuo Koike* Department of Chemical Pharmacology, Toho University School of Pharmaceutical Sciences, 2-2-1, Miyama, Funabashi, Chiba 274-8510, Japan Received July 19, 2000 Accepted September 29, 2000 ABSTRACT—The agonistic and antagonistic effects of (±)-pindolol (1-(1H-indol-4-yloxy)-3-[(1-methyleth- yl)amino]-2-propanol) were estimated to clarify whether (±)-pindolol acts as a partial agonist on atypical b-adrenoceptors in the guinea pig duodenum. (±)-Pindolol induced concentration-dependent relaxation with a pD2 value of 5.10 ± 0.03 and an intrinsic activity of 0.83 ± 0.03. However, the relaxations to (±)-pindolol were not antagonized by the non-selective b 1- and b 2-adrenoceptor antagonist (±)-propranolol (1 mM). In the presence of (±)-propranolol (1 mM), the non-selective b1-, b2- and b3-adrenoceptor antagonist (±)-bupranolol (30 mM) induced a rightward shift of the concentration-response curves for (±)-pindolol (apparent pA2 = 5.41 ± 0.06). In the presence of (±)-propranolol, (±)-pindolol (10 mM) weakly but significant- ly antagonized the relaxant effects to catecholamines ((-)-isoprenaline, (-)-noradrenaline and (-)-adrenaline), a selective b 3-adrenoceptor agonist BRL37344 ((R*,R*)-(±)-4-[2-[(2-(3-chlorophenyl)-2-hydroxyethyl) amino]propyl]phenoxyacetic acid sodium salt) and a non-conventional partial b 3-adrenoceptor agonist (±)-CGP12177A ([4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one] hydrochloride). These results demonstrate that (±)-pindolol possesses both agonistic and antagonistic effects on atypical b-adrenoceptors in the guinea pig duodenum. -

Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy
Japan. J. Pharmacol. 35, 319-326 (1984) 319 Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy Shigeru IKEDA, Hiroshi TAMAOKI, Masuo AKAHANE and Yoshifumi NEBASHI Central ResearchLaboratories, Kissei Pharmaceutical Co., Ltd. 19-48 Yoshino,Matsumoto 399-65, Japan Accepted April7, 1984 Abstract•\Ritodrine hydrochloride (ritodrine) is a beta2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10-9-1 0-6 M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100-1,000 times less than that of iso- proterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol> ritodrine>isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with pro- pranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors. -
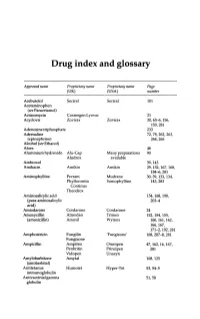
Drug Index and Glossary
Drug index and glossary Approved name Proprietary name Proprietary name Page (UK) (USA) number Acebutolol Sectral Sectral 101 Acetaminophen (see Paracetamol) Actinomycin Cosmegen Lyovac 21 Acyclovir Zovirax Zovirax 30, 65-6, 156, 159,281 Adenosine triphosphate 233 Adrenaline 72, 78, 262, 263, (epinephrine) 264,265 Alcohol (see Ethanol) Aloes 48 Aluminium hydroxide Alu-Cap Many preparations 90 Aludrox available Ambroxol 39,143 Amikacin Amikin Amikin 29,152,167,168, 184-6,281 Aminophylline Pecram Mudrane 30,39,133,134, Phyllocontin Somophylline 143,283 Continus Theodrox Aminosalicylic acid 154, 168, 198, (para-aminosalicylic 203-4 acid) Amiodarone Cordarone Cordarone 24 Amoxycillin Almodan Trlmox 152, 154, 155, (amoxicillin) Amoxil Wymox 160, 161, 162, 166,167, 171-2, 192, 281 Amphotericin Fungilin 'Fungizone' 168, 207--S, 281 Fungizone Ampicillin Ampifen Omnipen 47,162,16,167, Penbritin Principen 281 Vidopen Unasyn Amylobarbitone Amytal 108,125 (amobarbital) Antitetanus Humotet Hyper-Tet 53,54-5 immunoglobulin Antivaccinial gamma 51,58 globulin Drug index and glossary 289 Approved name Proprietary name Proprietary name Page (UK) (USA) number Antivaricella-zoster 65 immunoglobulin Apomorphine 97 Arnica 278 Ascorbic acid Redoxon Many preparations 70,75--6 available Aspirin Many preparations Many preparations 23,48,99,100, available available 141,272-3,276 Atebrin 12 (Atabrine, Medacrine, Quinacrine) Atenolol Tenormin Tenormin 101,120 Atropine Many combined Many combined 48,49,96,222, preparations preparations 234,250,26S-9 available available -

Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides
DRUGS Anesthesia/Anti-Convulsants: Carbamazepine Ethosuximide Halothane Lidocaine Phenytoin Valproic Acid Antibiotics: Aminoglycosides (Generic Examples: Gentamycin; Tobramycin; Amikacin) Amoxicillin Amphotericin B Ampicillin Azithromycin (A Special Macrolide) Ceftriaxone (Prototype 3rd Generation Cephalosporin) Chloramphenicol Chloroquine, Quinine and Mefloquine Ciprofloxacin (Prototype Fluoroquinolone) Clindamycin Demeclocycline (Another Tetracycline) Doxycycline (Prototype Tetracycline) Erythromycin (Prototype Macrolide) Indinavir Isoniazid Ketoconazole Metronidazole Nafcillin (Beta-Lactamase Resistant Penicillin) Praziquantel Rifampin Trimethoprim + Sulfamethoxazole Vancomycin Zidovudine Autonomic Drugs : Atropine Bethanechol Clonidine Cocaine Dobutamine Edrophonium Epinephrine Isoproteronol Labetalol (also Carvedilol) Methylphenidate Metoprolol Phenoxybenzamine Phentolamine Phenylepherine Physostigmine Pilocarpine Pindolol (also Acebutolol) Prazosin Propranolol Reserpine Ritodrine Blood D/O Drugs: Heparin Streptokinase TPA (Tissue Plasminogen Activator) Warfarin Cancer Drugs: 5-Fluoruracil + Folic Acid Bleomycin Busulfan Cisplatin Colchicine Cyclophoshamide Cyclosporine Dacarbazine Doxorubicin = Adriamycin Melphalan Methotrexate Vinchristine Cardiovascular Drugs: Amiodarone Captopril and Enalapril Digoxin Diltiazem Hydralazine Lidocaine Losartan Nifedipine Nitroglycerin and Isosorbide Dinitrate Nitroprusside Procainamide Quinidine Verapamil CNS Drugs: Alprazolam (Another Benzodiazepine) Desipramine (Secondary TCA) Diazepam (Prototype -
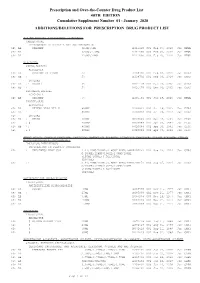
January 2020: Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 01 : January 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> AA XIROMED 325MG;5MG A 211690 001 Feb 07, 2020 Jan NEWA >A> AA 325MG;7.5MG A 211690 002 Feb 07, 2020 Jan NEWA >A> AA 325MG;10MG A 211690 003 Feb 07, 2020 Jan NEWA ACYCLOVIR CREAM;TOPICAL ACYCLOVIR >D> AB PERRIGO UK FINCO 5% A 208702 001 Feb 04, 2019 Jan CHRS >A> AB ! 5% A 208702 001 Feb 04, 2019 Jan CHRS ZOVIRAX >D> AB +! BAUSCH 5% N 021478 001 Dec 30, 2002 Jan CHRS >A> AB + 5% N 021478 001 Dec 30, 2002 Jan CHRS OINTMENT;TOPICAL ACYCLOVIR >A> AB XIROMED 5% A 201501 001 Jan 29, 2020 Jan NEWA TABLET;ORAL ACYCLOVIR >D> AB HETERO LABS LTD V 800MG A 203834 002 Oct 29, 2013 Jan CHRS >A> AB ! 800MG A 203834 002 Oct 29, 2013 Jan CHRS >D> ZOVIRAX >D> AB + MYLAN 400MG N 020089 001 Apr 30, 1991 Jan DISC >A> + @ 400MG N 020089 001 Apr 30, 1991 Jan DISC >D> AB +! 800MG N 020089 002 Apr 30, 1991 Jan DISC >A> + @ 800MG N 020089 002 Apr 30, 1991 Jan DISC AMINO ACIDS; CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM SULFATE; POTASSIUM CHLORIDE; SODIUM ACETATE; SODIUM GLYCEROPHOSPHATE; SOYBEAN OIL EMULSION;INTRAVENOUS PERIKABIVEN IN PLASTIC CONTAINER >D> + FRESENIUS KABI USA 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML ;105MG/100ML;3.5GM/100ML (2400ML) >A> +! 2.4%;20MG/100ML;6.8GM/100ML;68M N 200656 003 Aug 25, 2014 Jan CHRS G/100ML;124MG/100ML;170MG/100ML -

Multimedia Appendix 1. Search Strategy Developed for Medline Via Ovid to Identify Existing Systematics Review. Concept 1: BP
Multimedia Appendix 1. Search strategy developed for Medline via Ovid to identify existing systematics review. Concept 1: BP lowering regimens 1 exp antihypertensive agents/ 2 (antihypertensive$ adj (agent$ or drug)).tw. 3 exp thiazides/ 4 (chlorothiazide or benzothiadiazine or bendroflumethiazide or cyclopenthiazide or metolazone or xipamide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or polythiazide or trichlormethiazide or thiazide?).tw. 5 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodolin or thalitone or hygroton or indapamide or metindamide).tw. 6 ((loop or ceiling) adj diuretic?).tw. 7 (bumetanide or furosemide or torasemide).tw. 8 exp sodium potassium chloride symporter inhibitors/ 9 (eplerenone or amiloride or spironolactone or triamterene).tw. 10 or/1-9 11 exp angiotensin-converting enzyme inhibitors/ 12 ((angiotensin$ or kininase ii or dipeptidyl$) adj3 (convert$ or enzyme or inhibit$ or recept$)).tw. 13 (ace adj3 inhibit$).tw. 14 acei.tw. 15 exp enalapril/ 16 (alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril or teprotide).tw. 17 or/11-16 18 exp Angiotensin II Type 1 Receptor Blockers/ 19 (angiotensin$ adj4 receptor$ adj3 (antagon$ or block$)).tw. 20 exp losartan/ 21 (KT3-671 or candesartan or eprosartan or irbesartan or losartan or -

Adrenoceptor Agonists on Spontaneous Contractions of Human Nonpregnant Myometrium
PRACE ORYGINALNE Ginekol Pol. 2011, 82, 918-924 ginekologia Differences in the effects ofβ 2- and β3- adrenoceptor agonists on spontaneous contractions of human nonpregnant myometrium Odmienny wpływ agonistów receptorów β2- i β3-adrenergicznych na spontaniczne skurcze myometrium kobiet nieciężarnych Pędzińska-Betiuk Anna1*, Modzelewska Beata1, Jóźwik Marcin2, Jóźwik Maciej3, Kostrzewska Anna1 1 Department of Biophysics, Medical University of Białystok, Białystok, Poland 2 Department of Gynecology and Obstetrics, Kliniken Nordoberpfalz, Akademisches Lehrkrankenhaus der Universität Regensburg, Weiden, Germany 3 Department of Gynecology, Medical University of Białystok, Białystok, Poland * Current address: Department of Experimental Physiology and Pathophysiology, Medical University of Białystok, Białystok, Poland Abstract Objective: This study aimed to compare the relaxant properties of BRL 37344 with β2-adrenoceptors agonist ritodrine on the contractility of human nonpregnant myometrium. Material and methods: The activity of myometrial strips mounted in an organ bath was recorded under isometric conditions using force transducers with digital output. Contractility before and after cumulative additions of both uterorelaxants and with preincubation with β-adrenoceptor antagonists bupranolol, propranolol, and butoxamine were studied. Results: Both BRL 37344 (10-10 – 10-4 mol/L) and ritodrine (10-10 – 10-5 mol/L) decreased the area under curve, or AUC, value (logIC50 -6.45 ± 0.18 and -8.71 ± 0.35, respectively), and the degree of inhibition of spontaneous contractile activity was similar (< 30%). However, BRL 37344 decreased the mean frequency of contractions, whereas ritodrine decreased the mean amplitude of contractions. The inhibition of contractions by BRL 37344 was partially antagonized by bupranolol and propranolol, but not with butoxamine. The inhibition by ritodrine was counteracted by all these antagonists.