List Item Short-Acting Beta-Agonists Article-31 Referral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

18 December 2020 – to Date)
(18 December 2020 – to date) MEDICINES AND RELATED SUBSTANCES ACT 101 OF 1965 (Gazette No. 1171, Notice No. 1002 dated 7 July 1965. Commencement date: 1 April 1966 [Proc. No. 94, Gazette No. 1413] SCHEDULES Government Notice 935 in Government Gazette 31387 dated 5 September 2008. Commencement date: 5 September 2008. As amended by: Government Notice R1230 in Government Gazette 32838 dated 31 December 2009. Commencement date: 31 December 2009. Government Notice R227 in Government Gazette 35149 dated 15 March 2012. Commencement date: 15 March 2012. Government Notice R674 in Government Gazette 36827 dated 13 September 2013. Commencement date: 13 September 2013. Government Notice R690 in Government Gazette 36850 dated 20 September 2013. Commencement date: 20 September 2013. Government Notice R104 in Government Gazette 37318 dated 11 February 2014. Commencement date: 11 February 2014. Government Notice R352 in Government Gazette 37622 dated 8 May 2014. Commencement date: 8 May 2014. Government Notice R234 in Government Gazette 38586 dated 20 March 2015. Commencement date: 20 March 2015. Government Notice 254 in Government Gazette 39815 dated 15 March 2016. Commencement date: 15 March 2016. Government Notice 620 in Government Gazette 40041 dated 3 June 2016. Commencement date: 3 June 2016. Prepared by: Page 2 of 199 Government Notice 748 in Government Gazette 41009 dated 28 July 2017. Commencement date: 28 July 2017. Government Notice 1261 in Government Gazette 41256 dated 17 November 2017. Commencement date: 17 November 2017. Government Notice R1098 in Government Gazette 41971 dated 12 October 2018. Commencement date: 12 October 2018. Government Notice R1262 in Government Gazette 42052 dated 23 November 2018. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

Application Number
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 203975s000 SUMMARY REVIEW SUMMARY REVIEW OF REGULATORY ACTION Date: November 26, 2013 From: Badrul A. Chowdhury, MD, PhD Director, Division of Pulmonary, Allergy, and Rheumatology Products, CDER, FDA Subject: Division Director Summary Review NDA Number: 20-3975 Applicant Name: GlaxoSmithKline Date of Submission: December 18, 2012 PDUFA Goal Date: December 18, 2013 Proprietary Name: Anoro Ellipta Established Name: Umeclidinium and vilanterol Dosage form: Inhalation Powder (inhaler contains 2 double-foil blister strips, each with 30 blisters containing powder for oral inhalation) Strength: Umeclidinium 62.5 mcg per blister and vilanterol 25 mcg per blister Proposed Indications: Maintenance treatment of airflow obstruction in chronic obstructive pulmonary disease (COPD) Action: Approval 1. Introduction GlaxoSmithKline (GSK) submitted this 505(b)(1) new drug application for use of Anoro Ellipta (umeclidinium 62.5 mcg and vilanterol 25 mcg inhalation powder) for long-term once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD). The proposed dose is one inhalation (umeclidinium 62.5 mcg and vilanterol 25 mcg) once daily. The application is based on clinical efficacy and safety studies. This summary review will provide an overview of the application, with a focus on the clinical efficacy and safety studies. 2. Background There are several drug classes available for the relief of airflow obstruction in patients with COPD. These include short- and long-acting beta-2 adrenergic agonists, short- and long-acting anticholinergics, combination products containing beta-2 adrenergic agonists and anticholinergics, combination of long-acting beta-2 adrenergic agonists and corticosteroids, methylxanthines, and phosphodiesterase-4 (PDE4) inhibitors. -

Orciprenaline Sulphate (Alupent): Planned Withdrawal from the UK Market Following a Risk-Benefit Analysis
MHRA PUBLIC ASSESSMENT REPORT Orciprenaline sulphate (Alupent): planned withdrawal from the UK market following a risk-benefit analysis November 2009 Executive summary 2 1. Introduction 3 2. Summary of data 3 2.1 Clinical pharmacology 3 2.2 Efficacy 3 2.3 Safety 4 3. Conclusions 8 4. References 9 5. Glossary 10 1 EXECUTIVE SUMMARY (Please note that this summary is intended to be accessible to all members of the public, including health professionals) Background The Medicines and Healthcare products Regulatory Agency (MHRA) is the government agency responsible for regulating the effectiveness and safety of medicines and medical devices in the UK. We continually review the safety of all medicines in the UK, and inform healthcare professionals and the public of the latest safety updates. In our Public Assessment Reports, we discuss the evidence for a safety issue with a particular drug or drug class, and changes made to the product information for the drug on the basis of this evidence, which will help safeguard public health. This MHRA Public Assessment Report discusses a review of the risks and benefits of a medicine called orciprenaline sulphate. Orciprenaline sulphate is available for oral administration as a syrup used to treat reversible airways obstructiona, which is a symptom of asthmab and chronic obstructive pulmonary diseasec. It acts on specific areas in the body called β- receptors, which relaxes the muscles used for breathing and opens the airways in the lungs. Orciprenaline sulphate was licensed in 1972 and is marketed in the UK under the brand name Alupent Syrup. As with any medicine, the use of orciprenaline sulphate may lead to adverse reactions (side-effects) in some individuals, which are described in the product information, including the patient information leaflet (see the Electronic Medicines Compendium (product information) website). -

Case-Control Study Ofprescribed Fenoterol
170 Thorax 1990;45:170-175 Case-control study of prescribed fenoterol and death from asthma in New Zealand, 1977-81 Thorax: first published as 10.1136/thx.45.3.170 on 1 March 1990. Downloaded from N Pearce, J Grainger, M Atkinson, J Crane, C Burgess, C Culling, H Windom, R Beasley Abstract A recent New Zealand case-control study,' A previous New Zealand case-control conducted by our group, tested the hypothesis study of asthma deaths in the 5-45 year that the unsupervised self administration of age group during 1981-3 found that pres- fenoterol by inhalation increases the risk of cription of fenoterol (by metered dose death from asthma. This hypothesis arose inhaler) was associated with an increased primarily from epidemiological evidence link- risk of death in patients with severe ing the sale of fenoterol to the epidemic of asthma. One major criticism ofthis study deaths from asthma that New Zealand has was that drug data for the cases and experienced since the late 1970s,' 2 and controls came from different sources. A experimental evidence that fenoterol is a less new case-control design has been used to selective beta2 agonist than salbutamol, the evaluate the same hypothesis, with a dif- most commonly used beta agonist.3 The possi- ferent set of asthma deaths, the same ble mechanisms ofaction remain to be clarified, source for drug information being used but several potential mechanisms are apparent; for both cases and controls. This depen- these depend on whether the association is with ded on identifying deaths from asthma long term use of fenoterol or use in the acute during 1977-81 from national mortality attack. -

Hexoprenaline: Β-Adrenoreceptor Selectivity in Isolated Tissues from the Guinea-Pig
Clinical and Experimental Pharmacology and Physiology (1975) 2, 541-547. Hexoprenaline: p-adrenoreceptor selectivity in isolated tissues from the guinea-pig Stella R. O’Donnell and Janet C. Wanstall Department of Physiology, University of Queenstand, Brisbane, Australia (Received 12 March 1975; revision received 22 April 1975) SUMMARY 1. A catecholamine P-adrenoreceptor agonist, hexoprenaline, was examined in vitro on five guinea-pig tissues and its potency relative to isoprenaline (as 100) obtained. 2. Hexoprenaline clearly delineated between those tissues classified as containing P,-adrenoreceptors (trachea, hind limb blood vessels and uterus; relative potencies 219, 110 and 76 respectively) and those classified as containing P,-adrenoreceptors (atria and ileum; relative potencies 3.3 and 1.0 respectively). 3. Hexoprenaline differed from some previously studied noncatecholamine P-adrenoreceptor agonists in being only two-fold less potent, relative to isoprena- line, as a vasodilator in perfused hind limb than as a tracheal relaxant. Key words : j3-adrenoreceptors, blood vessels, bronchodilators, guinea-pig, hexo- prenaline, selectivity, trachea. INTRODUCTION In a previous study using in vitro preparations from the guinea-pig, O’Donnell & Wanstall (1974a) examined potential sympathomimetic bronchodilator compounds for their potency as tracheal relaxants (p,-adrenoreceptors), atrial stimulants (P,-adrenoreceptors) and vasodilators in the perfused hind limb (p2-adrenoreceptors). Some resorcinolamines showed selectivity for trachea compared with not only atria but also blood vessels. There is some evidence in dogs in vivo that other noncatecholamines, namely carbuterol (Wardell et al., 1974), and salbutamol and terbutaline (Wasserman & Levy, 1974), display similar select- ivity. If selectivity between respiratory and vascular smooth muscle represents selectivity at the p-adrenoreceptor level, it poses the question whether the P1/p2subclassification (Lands et al., 1967a; Lands, Luduena & Buzzo, 1967b) is too rigid. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -
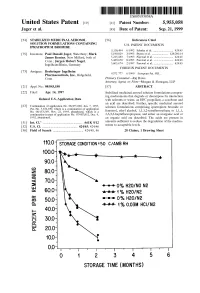
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy
Japan. J. Pharmacol. 35, 319-326 (1984) 319 Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy Shigeru IKEDA, Hiroshi TAMAOKI, Masuo AKAHANE and Yoshifumi NEBASHI Central ResearchLaboratories, Kissei Pharmaceutical Co., Ltd. 19-48 Yoshino,Matsumoto 399-65, Japan Accepted April7, 1984 Abstract•\Ritodrine hydrochloride (ritodrine) is a beta2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10-9-1 0-6 M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100-1,000 times less than that of iso- proterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol> ritodrine>isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with pro- pranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors. -
Drug Index and Glossary
Drug index and glossary Approved name Proprietary name Proprietary name Page (UK) (USA) number Acebutolol Sectral Sectral 101 Acetaminophen (see Paracetamol) Actinomycin Cosmegen Lyovac 21 Acyclovir Zovirax Zovirax 30, 65-6, 156, 159,281 Adenosine triphosphate 233 Adrenaline 72, 78, 262, 263, (epinephrine) 264,265 Alcohol (see Ethanol) Aloes 48 Aluminium hydroxide Alu-Cap Many preparations 90 Aludrox available Ambroxol 39,143 Amikacin Amikin Amikin 29,152,167,168, 184-6,281 Aminophylline Pecram Mudrane 30,39,133,134, Phyllocontin Somophylline 143,283 Continus Theodrox Aminosalicylic acid 154, 168, 198, (para-aminosalicylic 203-4 acid) Amiodarone Cordarone Cordarone 24 Amoxycillin Almodan Trlmox 152, 154, 155, (amoxicillin) Amoxil Wymox 160, 161, 162, 166,167, 171-2, 192, 281 Amphotericin Fungilin 'Fungizone' 168, 207--S, 281 Fungizone Ampicillin Ampifen Omnipen 47,162,16,167, Penbritin Principen 281 Vidopen Unasyn Amylobarbitone Amytal 108,125 (amobarbital) Antitetanus Humotet Hyper-Tet 53,54-5 immunoglobulin Antivaccinial gamma 51,58 globulin Drug index and glossary 289 Approved name Proprietary name Proprietary name Page (UK) (USA) number Antivaricella-zoster 65 immunoglobulin Apomorphine 97 Arnica 278 Ascorbic acid Redoxon Many preparations 70,75--6 available Aspirin Many preparations Many preparations 23,48,99,100, available available 141,272-3,276 Atebrin 12 (Atabrine, Medacrine, Quinacrine) Atenolol Tenormin Tenormin 101,120 Atropine Many combined Many combined 48,49,96,222, preparations preparations 234,250,26S-9 available available -

Addition of Intravenous Beta2-Agonists to Inhaled Beta2- Agonists for Acute Asthma (Review)
Addition of intravenous beta2-agonists to inhaled beta2- agonists for acute asthma (Review) Travers AH, Milan SJ, Jones AP, Camargo Jr CA, Rowe BH This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2012, Issue 12 http://www.thecochranelibrary.com Addition of intravenous beta2-agonists to inhaled beta2-agonists for acute asthma (Review) Copyright © 2012 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 3 BACKGROUND .................................... 5 OBJECTIVES ..................................... 5 METHODS ...................................... 5 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 12 AUTHORS’CONCLUSIONS . 12 ACKNOWLEDGEMENTS . 13 REFERENCES ..................................... 13 CHARACTERISTICSOFSTUDIES . 19 DATAANDANALYSES. 31 Analysis 1.1. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 1 Admissions. 31 Analysis 1.2. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 2 Length of stay. 32 Analysis 1.3. Comparison 1 IV + inhaled beta agonist vs. inhaled beta-agonist, Outcome 3 Pulse rate at 2