Division of Vital Statistics, Mortality Data Table Page 1 of 147
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
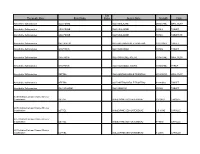
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Addictions and the Brain
9/18/2012 Addictions and the Brain TAAP Conference September 14, 2012 Acknowledgements • La Hacienda Treatment Center • American Society of Addiction Medicine • National Institute of Drug Abuse © 2012 La Hacienda Treatment Center. All rights reserved. 1 9/18/2012 Definition • A primary, progressive biochemical, psychosocial, genetically transmitted chronic disease of relapse who’s hallmarks are denial, loss of control and unmanageability. DSM IV Criteria for dependency: At least 3 of the 7 below 1. Withdrawal 2. Tolerance 3. The substance is taken in larger amounts or over a longer period than was intended. 4. There is a persistent desire or unsuccessful efforts to cut down or control substance use. 5. A great deal of time is spent in activities necessary to obtain the substance, use the substance, or recover from its effects. 6. Important social, occupational, or recreational activities are given up or reduced because of the substance use. 7. The substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance. © 2012 La Hacienda Treatment Center. All rights reserved. 2 9/18/2012 Dispute between behavior and disease Present understanding of the Hypothalamus location of the disease hypothesis. © 2012 La Hacienda Treatment Center. All rights reserved. 3 9/18/2012 © 2012 La Hacienda Treatment Center. All rights reserved. 4 9/18/2012 © 2012 La Hacienda Treatment Center. All rights reserved. 5 9/18/2012 Dispute regarding behavior versus disease © 2012 La Hacienda Treatment Center. All rights reserved. 6 9/18/2012 © 2012 La Hacienda Treatment Center. -

Anti-Inflammatory Effects of Amantadine and Memantine
Journal of Personalized Medicine Communication Anti-Inflammatory Effects of Amantadine and Memantine: Possible Therapeutics for the Treatment of Covid-19? Félix Javier Jiménez-Jiménez 1,* , Hortensia Alonso-Navarro 1 , Elena García-Martín 2 and José A. G. Agúndez 2 1 Section of Neurology, Hospital Universitario del Sureste, Arganda del Rey, E-28500 Madrid, Spain; [email protected] 2 University Institute of Molecular Pathology Biomarkers, UNEx. ARADyAL Instituto de Salud Carlos III, E-10071 Cáceres, Spain; [email protected] (E.G.-M.); [email protected] (J.A.G.A.) * Correspondence: [email protected]; Tel.: +34-636968395 Received: 2 October 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: We have reviewed current data on the anti-inflammatory effects of amantadine and memantine in clinical and in vivo models of inflammation, and we propose that these effects have potential interest for the treatment of the SARS-CoV-2 infection (COVID-19 disease). To that end, we performed a literature search using the PubMed Database from 1966 up to October 31 2020, crossing the terms “amantadine” and “memantine” with “inflammation” and “anti-inflammatory”. Amantadine and/or memantine have shown anti-inflammatory effects in chronic hepatitis C, in neuroinflammation induced by sepsis and by lipopolysaccharides, experimental models of multiple sclerosis, spinal cord injury, and respiratory diseases. Since the inflammatory response is one of the main pathogenetic mechanisms in the progression of the SARS-CoV-2 infection, anti-inflammatory effects of amantadine and memantine could be hypothetically useful in the treatment of this condition. This potential utility deserves further research. Keywords: amantadine; memantine; anti-inflammatory effects; SARS-Cov-2; COVID-19; therapy 1. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Pharmaceutical Manufacturing Formulations Liquid Products V () L UME Sarfaraz K
HANDBOOK OF Pharmaceutical Manufacturing Formulations Liquid Products V () L UME Sarfaraz K. Niazi CRC PRESS Boca Raton London New York Washington, D.C. Table of Contents PART I Regulatory and Manufacturing Guidance 1 Chapter 1 Current Good Manufacturing Practice Considerations in Liquid Manufacturing 3 I. Introduction 3 II. Facilities 3 III. Equipment 3 IV. Raw Materials 4 V. Compounding 4 VI. Microbiological Quality 4 VII. Oral Suspensions 5 VIII. Product Specifications 5 IX. Process Validation 5 X. Stability 5 XI. Packaging 6 Chapter 2 Stability Testing of New Drug Substances and Products 7 I. Introduction 7 II. Drug Substance 7 A. General Case 8 B. Drug Substances Intended for Storage in a Refrigerator 8 C. Drag Substances Intended for Storage in a Freezer 8 D. Drug Substances Intended for Storage below -20°C 9 HI. Drag Product 10 A. General Case •' B. Drag Products Packaged in Impermeable Containers 11 C. Drag Products Packaged in Semipermeable Containers 11 D. Drag Products Intended for Storage in a Refrigerator 12 E. Drag Products Intended for Storage in a Freezer 13 F. Drag Products Intended for Storage below -20"C 13 IV Glossary 14 References 1() Chapter 3 Container Closure Systems '7 I. Introduction '7 A. Definitions '7 B. Current Good Manufacturing Practice, the Consumer Product Safety Commission, and Requirements on Containers and Closures 17 C. Additional Considerations 17 II. Qualification and Quality Control of Packaging Components 18 A. Description 21 B. Information about Suitability 21 C. Stability Data (Packaging Concerns) 22 D. Inhalation Drag Products 23 E. Injection and Ophthalmic Drag Products 23 F. -

(12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis Et Al
USOO7803838B2 (12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis et al. (45) Date of Patent: Sep. 28, 2010 (54) COMPOSITIONS COMPRISING NEBIVOLOL 2002fO169134 A1 11/2002 Davis 2002/0177586 A1 11/2002 Egan et al. (75) Inventors: Eric Davis, Morgantown, WV (US); 2002/0183305 A1 12/2002 Davis et al. John O'Donnell, Morgantown, WV 2002/0183317 A1 12/2002 Wagle et al. (US); Peter Bottini, Morgantown, WV 2002/0183365 A1 12/2002 Wagle et al. (US) 2002/0192203 A1 12, 2002 Cho 2003, OOO4194 A1 1, 2003 Gall (73) Assignee: Forest Laboratories Holdings Limited 2003, OO13699 A1 1/2003 Davis et al. (BM) 2003/0027820 A1 2, 2003 Gall (*) Notice: Subject to any disclaimer, the term of this 2003.0053981 A1 3/2003 Davis et al. patent is extended or adjusted under 35 2003, OO60489 A1 3/2003 Buckingham U.S.C. 154(b) by 455 days. 2003, OO69221 A1 4/2003 Kosoglou et al. 2003/0078190 A1* 4/2003 Weinberg ...................... 514f1 (21) Appl. No.: 11/141,235 2003/0078517 A1 4/2003 Kensey 2003/01 19428 A1 6/2003 Davis et al. (22) Filed: May 31, 2005 2003/01 19757 A1 6/2003 Davis 2003/01 19796 A1 6/2003 Strony (65) Prior Publication Data 2003.01.19808 A1 6/2003 LeBeaut et al. US 2005/027281.0 A1 Dec. 8, 2005 2003.01.19809 A1 6/2003 Davis 2003,0162824 A1 8, 2003 Krul Related U.S. Application Data 2003/0175344 A1 9, 2003 Waldet al. (60) Provisional application No. 60/577,423, filed on Jun. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Treatment of Schizophrenia Course Director: Philip Janicak, M.D
S6735- Treatment of Schizophrenia Course Director: Philip Janicak, M.D. #APAAM2016 Saturday, May 14, 2016 Marriott Marquis - Marquis Ballroom D psychiatry.org/ annualmeetingS4637 ANNUAL MEETING May 14-18, 2016 • Atlanta Reference • Janicak PG, Marder SR, Tandon R, Goldman M (Eds.). Schizophrenia: Recent Advances in Diagnosis and Treatment. New York, NY: Springer; 2014. Schizophrenia: Recent Diagnostic Advances, Neurobiology, and the Neuropharmacology of Antipsychotic Drug Therapy Rajiv Tandon, MD Professor of Psychiatry University of Florida College of Medicine Gainesville, Florida Annual Meeting of the American Psychiatric Association New York, New York May 3–7, 2014 Disclosure Information MEMBER, WPA PHARMACOPSYCHIATRY SECTION MEMBER, DSM-5 WORKGROUP ON PSYCHOTIC DISORDERS A CLINICIAN AND CLINICAL RESEARCHER Pharmacological Treatment of Any Disease • Know the Disease that you are treating • Nature; Treatment targets; Treatment goals; • Know the Treatments at your disposal • What they do; How they compare; Costs; • Principles of Treatment • Measurement-based; Targeted; Individualized Program Outline • Nature and Definition of psychosis? • Clinical description • What is wrong in psychotic illness • Dimensions of Psychopathology • Neurobiological Abnormalities • Mechanisms underlying antipsychotic effects? • What contributes to Efficacy • Basis of Side-effect differences 5 Challenges in DSM-IV Construct of Psychotic Disorders ♦ Indistinct Boundaries ♦ With Other Disorders (eg., with OCD) ♦ Within Group of Psychotic Disorders (eg. between -

Psychiatric Medications in Behavioral Healthcarev5
Copyright © The University of South Florida, 2012 Psychiatric Medications in Behavioral Healthcare An Important Notice None of the pages in this tutorial are meant to be a replacement for professional help. The science of medicine is constantly changing, and these changes alter treatment and drug therapies as a result of what is learned through research and clinical experience. The author has relied on resources believed to be reliable at the time material was developed. However, there is always the possibility of human error or changes in medical science and neither the authors nor the University of South Florida can guarantee that all the information in this program is in every respect accurate or complete and they are not responsible for any errors or omissions or for the results obtained from the use of such information. Each person that reads this program is encouraged to confirm the information with other sources and understand that it not be interpreted as medical or professional advice. All medical information needs to be carefully reviewed with a health care provider. Course Objectives At the completion of this program participants should be able to: • Identify at 5 categories of medications used to treat the symptoms of psychiatric disorders, the therapeutic effects of medications in each category, and the side effects associated with medications in each category. • Identify at least 5 medications and the benefits of those medications as compared to the medications. • Identify at least 5 reasons that a person may stop taking medications or not take medications as prescribed. • Demonstrate your learned understanding of psychiatric medications by passing the combined post‐ tests. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V.