WHO Pharmaceuticals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modifying Antirheumatic Drugs in Active Rheumatoid Arthritis: a Japan Phase 3 Trial (HARUKA)
MODERN RHEUMATOLOGY 2020, VOL. 30, NO. 2, 239–248 https://doi.org/10.1080/14397595.2019.1639939 ORIGINAL ARTICLE Sarilumab monotherapy or in combination with non-methotrexate disease- modifying antirheumatic drugs in active rheumatoid arthritis: A Japan phase 3 trial (HARUKA) Hideto Kamedaa, Kazuteru Wadab, Yoshinori Takahashib, Owen Haginoc, Hubert van Hoogstratend, Neil Grahame and Yoshiya Tanakaf aDivision of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Toho University (Ohashi Medical Center), Tokyo, Japan; bSanofi K.K., Tokyo, Japan; cSanofi, Bridgewater, NJ, USA; dSanofi-Genzyme, Cambridge, MA, USA; eRegeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; fFirst Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Downloaded from https://academic.oup.com/mr/article/30/2/239/6299750 by guest on 01 October 2021 Japan, Kitakyushu, Japan ABSTRACT ARTICLE HISTORY Objectives: To determine long-term safety and efficacy of sarilumab as monotherapy or with non- Received 13 March 2019 methotrexate (MTX) conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) in Accepted 1 July 2019 Japanese patients with active rheumatoid arthritis (RA). KEYWORDS Methods: In this double-blind, randomized study (NCT02373202), patients received subcutaneous sari- Rheumatoid arthritis; lumab 150 mg q2w (S150) or 200 mg q2w (S200) as monotherapy or with non-MTX csDMARDs for 52 sarilumab; Japan; phase III; weeks. The primary endpoint was safety. anti-IL-6 receptor Results: Sixty-one patients received monotherapy (S150, n ¼ 30; S200, n ¼ 31) and 30 received combin- ation therapy (S150 þ csDMARDs, n ¼ 15; S200 þ csDMARDs, n ¼ 15). Rates of treatment-emergent adverse events (TEAEs) were 83.3%/90.3%/93.3%/86.7% for S150/S200/S150 þ csDMARDs/ S200 þ csDMARDs, respectively. -

Study Protocol);
PF-06651600 B7981006 Final Protocol Amendment 1, 26 October 2016 A PHASE 2A, RANDOMIZED, DOUBLE-BLIND, PARALLEL GROUP, PLACEBO-CONTROLLED, MULTI-CENTER STUDY TO ASSESS THE EFFICACY AND SAFETY PROFILE OF PF-06651600 IN SUBJECTS WITH MODERATE TO SEVERE ACTIVE RHEUMATOID ARTHRITIS WITH AN INADEQUATE RESPONSE TO METHOTREXATE Investigational Product Number: PF-06651600 Investigational Product Name: Not Applicable (N/A) United States (US) Investigational New 131274 Drug (IND) Number: European Clinical Trials Database 2016-002862-30 (EudraCT) Number: Protocol Number: B7981006 Phase: 2a Page 1 PF-06651600 B7981006 Final Protocol Amendment 1, 26 October 2016 Document History Document Version Date Summary of Changes and Rationale Amendment 1 26 October 2016 In the Schedule of Activities, and Section 7.2.12, added audiogram testing Rationale: To monitor for potential changes in hearing between baseline [between Visit 1 and Visit 2 (inclusive)] and at the end of the study [between Visit 7 and Visit 9 (inclusive)]. The following exclusion criteria has been added in Section 4.2: Have current or recent history of clinically significant severe or progressive hearing loss or auditory disease. Subjects with hearing aids will be allowed to enter the study provided their hearing impairment is considered controlled/ clinically stable. Rationale: This has been added to ensure that subject hearing for safety is fully evaluated prior to study entry. Several additional minor changes were made to protocol language for the purposes of clarification. Original protocol 31 August 2016 Not applicable (N/A) This amendment incorporates all revisions to date, including amendments made at the request of country health authorities and institutional review boards (IRBs)/ethics committees (ECs). -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

24/3 Pag 121 Ok X WENDY
Treatment continuation rate in relation to efficacy and toxicity in long-term therapy with low-dose methotrexate, sulfasalazine, and bucillamine in 1358 Japanese patients with rheumatoid arthritis M. Nagashima, T. Matsuoka, K. Saitoh, T. Koyama, O. Kikuchi, S. Yoshino Department of Joint Disease and Rheumatism, Nippon Medical School, Japan. Abstract Objective To evaluate the effectiveness of disease-modifying antirheumatic drugs, namely, methotrexate (MTX), sulfasalazine (SSZ) and bucillamine (BUC) at low-doses (4, 6 or 8mg MTX, 500 or1000mg SSZ, and 100 or 200 mg BUC) in 1358 patients with a follow-up of at least 12 months and more than 120 months. Methods Clinical assessments were based on the number of painful joints (NPJ) and that of swollen joints (NSJ), CRP level, erythrocyte sedimentation rate, rheumatoid factor level and morning stiffness before and after treatment. Results were evaluated on the basis of the duration of treatment for each drug with inefficacy or inadequate efficacy as one endpoint for discontinuation and adverse drug reactions (ADRs) as the other in single agent and combination ther- apy. The incidence and nature of ADRs in single and combination treatment are described. Results The effects of MTX, SSZ and BUC on clinical parameters were monitored over the first three months, and in partic- ular, NPJs and NSJs were found to decrease significantly during single agent MTX or BUC treatment over 108 months. CRP levels remained significantly improved for more than 120 months with MTX. In the single and combi- nation long-term treatments, continuation rate with inefficacy or inadequate efficacy as the end point achieved for each of the treatments were 83.1% for MTX, 76.0% for BUC, 68.5% for SSZ, and in the case of the combination treatments, these rates were 83.3% for MTX + BUC and 71.0% for MTX+SSZ. -

Actemra® (Tocilizumab)
Actemra® (tocilizumab) (Intravenous) Document Number: MODA-0002 Last Review Date: 10/26/2020 Date of Origin: 09/21/2010 Dates Reviewed: 12/2010, 03/2011, 05/2011, 06/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 09/2012, 11/2012, 12/2012, 03/2013, 06/2013, 09/2013, 11/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 05/2015, 09/2015, 12/0215, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 05/2017, 09/2017, 12/2017, 03/2018, 06/2018, 10/2018, 10/2019, 10/2020, 11/2020 I. Length of Authorization Coverage will be provided as follows: o Castleman’s Disease: 4 months and may be renewed o Cytokine Release Syndrome: 4 doses only and may not be renewed o Immune Checkpoint Inhibitor related arthritis: 1 dose and may not be renewed o All other indications: 6 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: o Actemra 80 mg/4 mL vial: 1 vial per 14 days o Actemra 200 mg/10 mL vial: 1 vial per 14 days o Actemra 400 mg/20 mL vial: 2 vials per 14 days B. Max Units (per dose and over time) [HCPCS Unit]: Diagnosis Billable Units Interval (days) Rheumatoid Arthritis & Polyarticular Juvenile Idiopathic 800 28 Arthritis, NMOSD Systemic Juvenile Idiopathic Arthritis, Castleman’s Disease (NHL) & Acute Graft Versus Host Disease 800 14 (aGVHD) Cytokine Release Syndrome (CRS) 3200 1 course of therapy only Immune Checkpoint Inhibitor related arthritis 800 1 course of therapy only III. -

Pharmacologyonline 2: 971-1020 (2009) Newsletter Gabriella Galizia
Pharmacologyonline 2: 971-1020 (2009) Newsletter Gabriella Galizia THE TREATMENT OF THE SCHIZOPHRENIA: AN OVERVIEW Gabriella Galizia School of Pharmacy,University of Salerno, Italy e-mail: [email protected] Summary The schizophrenia is a kind of psychiatric disease, characterized by a course longer than six months (usually chronic or relapsing), by the persistence of symptoms of alteration of mind, behaviour and emotion, with such a seriousness to limitate the normal activity of a person. The terms antipsychotic and neuroleptic define a group of medicine principally used to treat schizophrenia, but they are also efficacious for other psychosis and in states of psychic agitation. The antipsychotics are divided into two classes: classic or typical and atypical. The paliperidone, the major metabolite of risperidone, shares with the native drug the characteristics of receptoral bond and of antagonism of serotonin (5HT2A) and dopamine (D2). It's available in a prolonged release formulation and it allows the administration once daily. Besides, the paliperidone has a pharmacological action independent of CYT P450 and in such way a lot of due pharmacological interactions would be avoided to interference with the activity of the CYP2D6, that is known to have involved in the metabolism of the 25% of the drugs of commune therapeutic employment. Introduction The schizophrenia has been a very hard disease to investigate by the research. This is not surprising because it involves the most mysterious aspects of human mind, as emotions and cognitive processes. According to scientific conventions, the schizophrenia is a kind of psychiatric disease, characterized by a course longer than six months (usually chronic or relapsing), by the persistence of symptoms of alteration of mind, behaviour and emotion, with such a seriousness to limitate the normal activity of a person. -

Actemra (Tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA
Policy Title: Actemra (tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA Effective Date: 01/01/2020 Review Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Revision Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Purpose: To support safe, effective and appropriate use of Actemra (tocilizumab). Scope: Medicaid, Commerical, Medicare-Medicaid Plan (MMP) Policy Statement: Actemra (tocilizumab) is covered under the Medical Benefit when used within the following guidelines for non-oncology indications. Use outside of these guidelines may result in non-payment unless approved under an exception process. For oncology indications, please refer to NHPRI Oncology Policy Procedure: Coverage of Actemra (tocilizumab) will be reviewed prospectively via the prior authorization process based on criteria below. Initial Criteria: Patient has been evaluated and screened for the presence of latent TB infection prior to initiating treatment; AND Patient does not have an active infection, including clinically important localized infections; AND Must not be administered concurrently with live vaccines; AND Patient is not on concurrent treatment with another TNF-inhibitor, biologic response modifier or other non-biologic agent (i.e., apremilast, tofacitinib, baricitinib); MMP members who have previously received this medication within the past 365 days are not subject to Step Therapy Requirements Rheumatoid Arthritis Patient is 18 years or older; AND Physician has assessed baseline disease severity utilizing an objective measure/tool; AND -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -
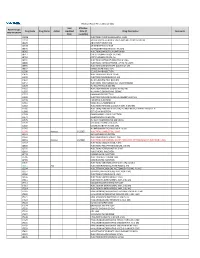
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -

Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy
Japan. J. Pharmacol. 35, 319-326 (1984) 319 Effects of Ritodrine Hydrochloride, a Beta2-Adrenoceptor Stimulant, on Uterine Motilities in Late Pregnancy Shigeru IKEDA, Hiroshi TAMAOKI, Masuo AKAHANE and Yoshifumi NEBASHI Central ResearchLaboratories, Kissei Pharmaceutical Co., Ltd. 19-48 Yoshino,Matsumoto 399-65, Japan Accepted April7, 1984 Abstract•\Ritodrine hydrochloride (ritodrine) is a beta2-adrenoceptor stimulant which has been effectively prescribed for the prevention of premature labor. The present studies were carried out to investigate the effects of ritodrine on uterine motility in rats and rabbits during gestation, as compared with those of isoproterenol and isoxsuprine. The results were as follows: 1) Spontaneous movements and evoked contractile responses of isolated rat uterus (19-20th days of gestation) were suppressed by 10-9-1 0-6 M ritodrine. The potency of ritodrine was approximately 10 times more than that of isoxsuprine and 100-1,000 times less than that of iso- proterenol. 2) When these drugs were administered to pregnant rats or rabbits intravenously, the tocolytic potency was in the following order: isoproterenol> ritodrine>isoxsuprine. 3) Ritodrine induced hypotension and tachycardia, but these effects were less than those of isoproterenol and isoxsuprine. 4) The effects of isoproterenol and ritodrine were almost prevented by pretreatment with pro- pranolol, but those of isoxsuprine were only partially or not affected. These results suggest that ritodrine is effective in preventing the uterine contractions in rats and rabbits and that it has less effect on the circulatory system than isoproterenol and isoxsuprine. It is also concluded that ritodrine produces these effects through activation of beta-adrenoceptors.