Actemra (Tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actemra® (Tocilizumab)
Actemra® (tocilizumab) (Intravenous) Document Number: MODA-0002 Last Review Date: 10/26/2020 Date of Origin: 09/21/2010 Dates Reviewed: 12/2010, 03/2011, 05/2011, 06/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 09/2012, 11/2012, 12/2012, 03/2013, 06/2013, 09/2013, 11/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 05/2015, 09/2015, 12/0215, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 05/2017, 09/2017, 12/2017, 03/2018, 06/2018, 10/2018, 10/2019, 10/2020, 11/2020 I. Length of Authorization Coverage will be provided as follows: o Castleman’s Disease: 4 months and may be renewed o Cytokine Release Syndrome: 4 doses only and may not be renewed o Immune Checkpoint Inhibitor related arthritis: 1 dose and may not be renewed o All other indications: 6 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: o Actemra 80 mg/4 mL vial: 1 vial per 14 days o Actemra 200 mg/10 mL vial: 1 vial per 14 days o Actemra 400 mg/20 mL vial: 2 vials per 14 days B. Max Units (per dose and over time) [HCPCS Unit]: Diagnosis Billable Units Interval (days) Rheumatoid Arthritis & Polyarticular Juvenile Idiopathic 800 28 Arthritis, NMOSD Systemic Juvenile Idiopathic Arthritis, Castleman’s Disease (NHL) & Acute Graft Versus Host Disease 800 14 (aGVHD) Cytokine Release Syndrome (CRS) 3200 1 course of therapy only Immune Checkpoint Inhibitor related arthritis 800 1 course of therapy only III. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -
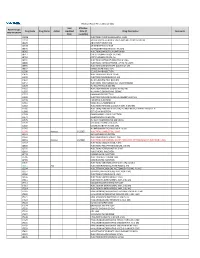
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

NICE UPDATE for COMMISSIONERS April 2019
South, Central and West NICE UPDATE FOR COMMISSIONERS April 2019 This NICE Update for Commissioners includes: At-a-glance summary Headline update: what’s been published? Guidance and quality standards published by NICE in March 2019 What’s new for CCGs? Horizon scanning What’s coming out from NICE in the next six months? For your reference, a summary of the types of NICE guidance Reference – a guide to NICE products The next (May 2019) NICE Update for Commissioners will be issued at the beginning of June 2019. For further information about NICE guidance and its implementation contact: Tiina Korhonen, Clinical Effectiveness Lead Kathryn Markey, Clinical Effectiveness Manager Kate Forbes, Clinical Effectiveness Manager Rebecca Hodge, Clinical Effectiveness Manager Gill Barlow, Clinical Effectiveness Manager Katie Newens, Clinical Effectiveness Researcher Rachel Finch, Clinical Effectiveness Administrator [email protected] 1 | p a g e At-a-glance summary The table below shows ALL NICE guidance published in April2019. Those likely to have significant impact for CCG commissioners are discussed further in the ‘What’s new for Clinical Commissioning Groups’ section (link to relevant section provided within guidance reference). Guidance type and Title Commissioner(s) Main providers(s) Impact for CCG commissioners (financial /public reference interest/quality of care) Technology Appraisal – Daratumumab with NHS England Secondary care - TA573 bortezomib and acute and dexamethasone for Tertiary care previously treated multiple myeloma Technology Appraisal – Certolizumab pegol for CCGs Primary care, NICE does not expect this guidance to have a TA574 treating moderate to severe secondary care - significant impact on resources; that is, it will be plaque psoriasis acute and tertiary less than £5 million per year in England (or care £9,100 per 100,000 population) because the technology is an option alongside current standard treatment options and is available at a similar price. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

CDER Therapeutic Biologic Products List
CDER Therapeutic Biologic Products This list is intended to include all the Center for Drug Evaluation and Research (CDER) user fee billable therapeutic biological products and potencies approved under Section 351 of the Public Health Service Act. The Orange Book includes a section entitled "Drug Products with Approval under Section 505 of the Act Administered by CBER." Included on that list are several products that have been transferred to CDER which would be considered billable also. Program fees are assessed for each potency in which the approved (non-revoked, non-suspended) product is manufactured in final dosage form. When evaluating the specific strength or potency of a drug in final dosage form for purposes of assessing program fees for liquid parenteral biological products, CDER intends to take into consideration both the total amount of drug substance in mass or units of activity in a product and the concentration of drug substance (mass or units of activity per unit volume of product). Biologic products considered to have a different strength or potency in a final dosage form will be given separate entries in the Biologics List and assessed separate program fees. An auto-injector that has the same strength or potency as a prefilled syringe or vial will generally be assessed a separate prescription drug program fee. In certain circumstances, products which have been discontinued from marketing but are still licensed are not assessed program fees. Those products are identified on the CDER Discontinued Biologic Product List section. The potency information contained in this list is based on information in our database. -

Avelumab) Name
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: 761049Orig1s009 Trade Name: Bavencio injection, for intravenous use Generic or Proper (avelumab) Name: Sponsor: EMD Serono Approval Date: June 30, 2020 Indication: For the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed with first-line platinum-containing chemotherapy. CENTER FOR DRUG EVALUATION AND RESEARCH 761049Orig1s009 CONTENTS Reviews / Information Included in this NDA Review. Approval Letter X Other Action Letters Labeling X REMS Officer/Employee List Multidiscipline Review(s) X • Summary Review • Office Director • Cross Discipline Team Leader • Clinical • Non-Clinical • Statistical • Clinical Pharmacology • Clinical Microbiology/Virology Product Quality Review(s) X Other Reviews X Risk Assessment and Risk Mitigation Review(s) Proprietary Name Review(s) Administrative/Correspondence Document(s) CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 761049Orig1s009 APPROVAL LETTER BLA 761049/S-009 SUPPLEMENT APPROVAL/ FULFILLMENT OF POSTMARKETING REQUIREMENT EMD Serono, Inc. Attention: Jennifer L. Stevens, JD Executive Director US Hub Lead/Global Regulatory Program Lead 45A Middlesex Turnpike Billerica, MA 01821 Dear Ms. Stevens: Please refer to your supplemental biologics license application dated April 7, 2020, received April 7, 2020, and your amendments, submitted under section 351(a) of the Public Health Service Act for Bavencio (avelumab) Injection. This Prior Approval supplemental biologics application provides for a new indication for the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed with first-line platinum-containing chemotherapy. We also refer to your biologics license application (BLA) 761078, approved May 9, 2017, under the regulations at 21 CFR 601 Subpart E for Accelerated Approval of Biological Products for Serious or Life-Threatening Illnesses. -

Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer
cancers Review Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer Antonio Lopez-Beltran 1,*,† , Alessia Cimadamore 2,† , Ana Blanca 3, Francesco Massari 4 , Nuno Vau 5, Marina Scarpelli 2, Liang Cheng 6 and Rodolfo Montironi 2,* 1 Unit of Anatomic Pathology, Department of Morphological Sciences, Cordoba University Medical School, 14004 Cordoba, Spain 2 Pathological Anatomy, School of Medicine, United Hospitals, Polytechnic University of the Marche Region, 60126 Ancona, Italy; [email protected] (A.C.); [email protected] (M.S.) 3 Maimonides Biomedical Research Institute of Cordoba, Department of Urology, University Hospital of Reina Sofia, 14004 Cordoba, Spain; [email protected] 4 Division of Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, 40138 Bologna, Italy; [email protected] 5 Medical Oncology, Champalimaud Clinical Center, 1400-038 Lisbon, Portugal; [email protected] 6 Department of Pathology and Laboratory Medicine, School of Medicine, Indiana University, Indianapolis, IN 46202, USA; [email protected] * Correspondence: [email protected] or [email protected] (A.L.-B.); [email protected] (R.M.); Tel.: +34-9-5721-8992 (A.L.-B.); +39-0-71-596-4830 (R.M.); Fax: +34-9-5721-8229 (A.L.-B.) † These authors contributed equally to the work. Simple Summary: In this review, we examined relevant clinical trial results with immune check- point inhibitors in patients with metastatic urothelial cancer. We also focused on the potential of immunotherapy in the adjuvant and neoadjuvant setting or as part of drug combinations. Finally, we briefly review the current landscape of biomarkers of response to immune checkpoint inhibitors, such as programmed death-ligand 1 (PD-L1) expression, tumor mutation burden, molecular subtypes Citation: Lopez-Beltran, A.; of bladder cancer, and immune-gene expression profiling. -

Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology
molecules Review Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology Hyun Tae Lee , Sang Hyung Lee and Yong-Seok Heo * Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea; [email protected] (H.T.L.); [email protected] (S.H.L.) * Correspondence: [email protected]; Tel.: +82-2-450-3408; Fax: +82-2-3436-5382 Academic Editor: Silvano Geremia Received: 4 March 2019; Accepted: 22 March 2019; Published: 26 March 2019 Abstract: Cancer cells can evade immune surveillance through the molecular interactions of immune checkpoint proteins, including programmed death 1 (PD-1), PD-L1, and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Since 2011, the FDA-approved antibody drugs ipilimumab (Yervoy®), nivolumab (Opdivo®), pembrolizumab (Keytruda®), cemiplimab (Libtayo®), atezolizumab (Tecentriq®), durvalumab (Imfinzi®), and avelumab (Bavencio®), which block the immune checkpoint proteins, have brought about a significant breakthrough in the treatment of a wide range of cancers, as they can induce durable therapeutic responses. In recent years, crystal structures of the antibodies against PD-1, PD-L1, and CTLA-4 have been reported. In this review, we describe the latest structural studies of these monoclonal antibodies and their interactions with the immune checkpoint proteins. A comprehensive analysis of the interactions of these immune checkpoint blockers can provide a better understanding of their therapeutic mechanisms of action. The accumulation of these structural studies would provide a basis that is essential for the rational design of next-generation therapies in immuno-oncology. Keywords: crystal structure; immune checkpoint; PD-1; PD-L1; CTLA-4; cancer; therapeutic antibody 1. -

Product Monograph
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION IMFINZI® durvalumab for injection concentrate for solution for infusion, 50 mg / mL, intravenous antineoplastic agent, monoclonal antibody IMFINZI (durvalumab) indicated for the treatment of patients with: Locally advanced or metastatic urothelial carcinoma who: o Have disease progression during or following platinum-containing chemotherapy o Have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy has been issued marketing authorization with conditions, pending the results of trials to verify its clinical benefit. Patients should be advised of the nature of the authorization. For further information for IMFINZI, please refer to Health Canada’s Notice of Compliance with conditions - drug products web site: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices- avis/conditions/index-eng.php. IMFINZI has been issued marketing authorization without conditions for: Treatment of patients with locally advanced, unresectable, Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following platinum-based chemoradiation therapy First-line treatment of adult patients with extensive-stage small cell lung cancer (ES- SCLC) in combination with etoposide and either carboplatin or cisplatin AstraZeneca Canada Inc. Date of Initial Approval: 1004 Middlegate Road, Suite 5000 November 3, 2017 Mississauga, Ontario L4Y 1M4 www.astrazeneca.ca Date of Revision: March 30, 2021 Submission Control No: 243395 IMFINZI® Product Monograph. COPYRIGHT 2017-2021, ASTRAZENECA CANADA INC. Page 1 of 55 This product has been authorized under the Notice of Compliance with Conditions (NOC/c) for one or all of its indicated uses. What is a Notice of Compliance with Conditions (NOC/c)? An NOC/c is a form of market approval granted to a product on the basis of promising evidence of clinical effectiveness following review of the submission by Health Canada. -

Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody–Drug Conjugate, in Relapsed/ Refractory B-Cell Non-Hodgkin Lymphoma Brad S
Published OnlineFirst November 4, 2019; DOI: 10.1158/1078-0432.CCR-19-0711 Clinical Trials: Targeted Therapy Clinical Cancer Research A Phase I Study of ADCT-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody–Drug Conjugate, in Relapsed/ Refractory B-Cell Non-Hodgkin Lymphoma Brad S. Kahl1, Mehdi Hamadani2, John Radford3, Carmelo Carlo-Stella4, Paolo Caimi5, Erin Reid6, Jay M. Feingold7, Kirit M. Ardeshna8, Melhem Solh9, Leonard T. Heffner10, David Ungar7, Shui He7, Joseph Boni7, Karin Havenith11, and Owen A. O'Connor12 Abstract Purpose: ADCT-402 (loncastuximab tesirine) is an anti- 200 mg/kg. Treatment-emergent adverse events (TEAEs) were body–drug conjugate comprising a CD19-targeting antibody experienced by 87/88 (98.9%) patients. Most common TEAEs and pyrrolobenzodiazepine dimers. A first-in-human study (20% of patients) were hematologic abnormalities, fatigue, evaluated the safety and preliminary clinical activity of lon- edema, liver test abnormalities, nausea, rash, and dyspnea. castuximab tesirine in patients with B-cell non-Hodgkin lym- Grade 3 TEAEs (5% of patients) included hematologic phoma (NHL). abnormalities, liver test abnormalities, fatigue, and dyspnea. Experimental Design: A multicenter, phase I, dose- Overall response rate at doses 120 mg/kg was 59.4% (41 of 69 escalation and dose-expansion study enrolled patients ages patients; 40.6% complete response; 18.8% partial response). 18 years with relapsed/refractory (R/R) B-cell NHL. Patients Median duration of response, progression-free survival, and received loncastuximab tesirine every 3 weeks at doses overall survival (all doses) were 4.8, 5.5, and 11.6 months, assigned by a 3þ3 dose-escalation design. -

Rxoutlook® 4Th Quarter 2020
® RxOutlook 4th Quarter 2020 optum.com/optumrx a RxOutlook 4th Quarter 2020 While COVID-19 vaccines draw most attention, multiple “firsts” are expected from the pipeline in 1Q:2021 Great attention is being given to pipeline drugs that are being rapidly developed for the treatment or prevention of SARS- CoV-19 (COVID-19) infection, particularly two vaccines that are likely to receive emergency use authorization (EUA) from the Food and Drug Administration (FDA) in the near future. Earlier this year, FDA issued a Guidance for Industry that indicated the FDA expected any vaccine for COVID-19 to have at least 50% efficacy in preventing COVID-19. In November, two manufacturers, Pfizer and Moderna, released top-line results from interim analyses of their investigational COVID-19 vaccines. Pfizer stated their vaccine, BNT162b2 had demonstrated > 90% efficacy. Several days later, Moderna stated their vaccine, mRNA-1273, had demonstrated 94% efficacy. Many unknowns still exist, such as the durability of response, vaccine performance in vulnerable sub-populations, safety, and tolerability in the short and long term. Considering the first U.S. case of COVID-19 was detected less than 12 months ago, the fact that two vaccines have far exceeded the FDA’s guidance and are poised to earn EUA clearance, is remarkable. If the final data indicates a positive risk vs. benefit profile and supports final FDA clearance, there may be lessons from this accelerated development timeline that could be applied to the larger drug development pipeline in the future. Meanwhile, drug development in other areas continues. In this edition of RxOutlook, we highlight 12 key pipeline drugs with potential to launch by the end of the first quarter of 2021.