Minutes of the CHMP Meeting 9-12 November 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Actemra® (Tocilizumab)
Actemra® (tocilizumab) (Intravenous) Document Number: MODA-0002 Last Review Date: 10/26/2020 Date of Origin: 09/21/2010 Dates Reviewed: 12/2010, 03/2011, 05/2011, 06/2011, 09/2011, 12/2011, 03/2012, 06/2012, 09/2012, 09/2012, 11/2012, 12/2012, 03/2013, 06/2013, 09/2013, 11/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 05/2015, 09/2015, 12/0215, 03/2016, 06/2016, 09/2016, 12/2016, 03/2017, 05/2017, 09/2017, 12/2017, 03/2018, 06/2018, 10/2018, 10/2019, 10/2020, 11/2020 I. Length of Authorization Coverage will be provided as follows: o Castleman’s Disease: 4 months and may be renewed o Cytokine Release Syndrome: 4 doses only and may not be renewed o Immune Checkpoint Inhibitor related arthritis: 1 dose and may not be renewed o All other indications: 6 months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: o Actemra 80 mg/4 mL vial: 1 vial per 14 days o Actemra 200 mg/10 mL vial: 1 vial per 14 days o Actemra 400 mg/20 mL vial: 2 vials per 14 days B. Max Units (per dose and over time) [HCPCS Unit]: Diagnosis Billable Units Interval (days) Rheumatoid Arthritis & Polyarticular Juvenile Idiopathic 800 28 Arthritis, NMOSD Systemic Juvenile Idiopathic Arthritis, Castleman’s Disease (NHL) & Acute Graft Versus Host Disease 800 14 (aGVHD) Cytokine Release Syndrome (CRS) 3200 1 course of therapy only Immune Checkpoint Inhibitor related arthritis 800 1 course of therapy only III. -

Letter to FDA on Review of Biogen's Drug
June 2, 2021 Janet Woodcock, M.D. Acting Commissioner of Food and Drugs Food and Drug Administration 10903 New Hampshire Ave Silver Spring, MD 20993-0002 RE: Food and Drug Administration’s Review of Biogen’s drug Aducanumab for Alzheimer’s disease Dear Acting Commissioner Woodcock: The American Geriatrics Society (AGS), an organization dedicated to improving the health and quality of life of all older adults, is writing to express our concern that the Food and Drug Administration’s (FDA) upcoming review and potential approval of Aducanumab for use in treating patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) is premature given the lack of sufficient evidence to support that Aducanumab reduces progression of Alzheimer’s disease and that the potential benefits as a treatment for patients with MCI and AD could outweigh the potential harms. The AGS is a not-for-profit organization comprised of nearly 6,000 geriatrics health professionals who are devoted to improving the health, independence, and quality of life of all older adults. Our members include geriatricians, geriatrics nurse practitioners, social workers, family practitioners, physician assistants, pharmacists, and internists who are pioneers in advanced-illness care for older individuals, with a focus on championing interprofessional teams, eliciting personal care goals, and treating older people as whole persons. We provide leadership to healthcare professionals, policymakers, and the public by implementing and advocating for programs in patient care, research, professional and public education, and public policy. We are familiar with the information that has been released to date. Aducanumab, a human monoclonal antibody developed by Biogen, was assessed in two identical phase III randomized controlled trials, ENGAGE and EMERGE, planned to provide 18-month outcome data in patients with MCI and AD, all with positive amyloid PET scans. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

Horizon Scanning Status Report June 2019
Statement of Funding and Purpose This report incorporates data collected during implementation of the Patient-Centered Outcomes Research Institute (PCORI) Health Care Horizon Scanning System, operated by ECRI Institute under contract to PCORI, Washington, DC (Contract No. MSA-HORIZSCAN-ECRI-ENG- 2018.7.12). The findings and conclusions in this document are those of the authors, who are responsible for its content. No statement in this report should be construed as an official position of PCORI. An intervention that potentially meets inclusion criteria might not appear in this report simply because the horizon scanning system has not yet detected it or it does not yet meet inclusion criteria outlined in the PCORI Health Care Horizon Scanning System: Horizon Scanning Protocol and Operations Manual. Inclusion or absence of interventions in the horizon scanning reports will change over time as new information is collected; therefore, inclusion or absence should not be construed as either an endorsement or rejection of specific interventions. A representative from PCORI served as a contracting officer’s technical representative and provided input during the implementation of the horizon scanning system. PCORI does not directly participate in horizon scanning or assessing leads or topics and did not provide opinions regarding potential impact of interventions. Financial Disclosure Statement None of the individuals compiling this information have any affiliations or financial involvement that conflicts with the material presented in this report. Public Domain Notice This document is in the public domain and may be used and reprinted without special permission. Citation of the source is appreciated. All statements, findings, and conclusions in this publication are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI) or its Board of Governors. -

Actemra (Tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA
Policy Title: Actemra (tocilizumab) NON-ONCOLOGY POLICY (Intravenous) Department: PHA Effective Date: 01/01/2020 Review Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Revision Date: 09/25/2019, 12/18/2019, 1/22/2020, 8/3/2020 Purpose: To support safe, effective and appropriate use of Actemra (tocilizumab). Scope: Medicaid, Commerical, Medicare-Medicaid Plan (MMP) Policy Statement: Actemra (tocilizumab) is covered under the Medical Benefit when used within the following guidelines for non-oncology indications. Use outside of these guidelines may result in non-payment unless approved under an exception process. For oncology indications, please refer to NHPRI Oncology Policy Procedure: Coverage of Actemra (tocilizumab) will be reviewed prospectively via the prior authorization process based on criteria below. Initial Criteria: Patient has been evaluated and screened for the presence of latent TB infection prior to initiating treatment; AND Patient does not have an active infection, including clinically important localized infections; AND Must not be administered concurrently with live vaccines; AND Patient is not on concurrent treatment with another TNF-inhibitor, biologic response modifier or other non-biologic agent (i.e., apremilast, tofacitinib, baricitinib); MMP members who have previously received this medication within the past 365 days are not subject to Step Therapy Requirements Rheumatoid Arthritis Patient is 18 years or older; AND Physician has assessed baseline disease severity utilizing an objective measure/tool; AND -

Treatment of Alzheimer's Disease and Blood–Brain Barrier Drug Delivery
pharmaceuticals Review Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery William M. Pardridge Department of Medicine, University of California, Los Angeles, CA 90024, USA; [email protected] Received: 24 October 2020; Accepted: 13 November 2020; Published: 16 November 2020 Abstract: Despite the enormity of the societal and health burdens caused by Alzheimer’s disease (AD), there have been no FDA approvals for new therapeutics for AD since 2003. This profound lack of progress in treatment of AD is due to dual problems, both related to the blood–brain barrier (BBB). First, 98% of small molecule drugs do not cross the BBB, and ~100% of biologic drugs do not cross the BBB, so BBB drug delivery technology is needed in AD drug development. Second, the pharmaceutical industry has not developed BBB drug delivery technology, which would enable industry to invent new therapeutics for AD that actually penetrate into brain parenchyma from blood. In 2020, less than 1% of all AD drug development projects use a BBB drug delivery technology. The pathogenesis of AD involves chronic neuro-inflammation, the progressive deposition of insoluble amyloid-beta or tau aggregates, and neural degeneration. New drugs that both attack these multiple sites in AD, and that have been coupled with BBB drug delivery technology, can lead to new and effective treatments of this serious disorder. Keywords: blood–brain barrier; brain drug delivery; drug targeting; endothelium; Alzheimer’s disease; therapeutic antibodies; neurotrophins; TNF inhibitors 1. Introduction Alzheimer’s Disease (AD) afflicts over 50 million people world-wide, and this health burden costs over 1% of global GDP [1]. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -

Medical Drug and Step Therapy Prior Authorization List for Medicare Plus Bluesm and BCN Advantagesm Members Revised September 2021
Medical Drug and Step Therapy Prior Authorization List for Medicare Plus BlueSM and BCN AdvantageSM members Revised September 2021 This document lists the medical benefit drugs that have authorization or step therapy requirements for Medicare Advantage members. See the revision history at the end of this document for information about changes to this list. Prior authorization Submit authorization requirement effective date request through AIM HCPCS Step therapy Medicare BCN Specialty codes Generic name Trade name requirement Plus Blue Advantage NovoLogix® Health® C9076 Lisocabtagene maraleucel Breyanzi® 2/11/2021 2/11/2021 C9078 Trilaciclib Cosela™ 5/24/2021 5/24/2021 C9079 Evinacumab-dgnb Evkeeza™ 6/22/2021 6/22/2021 C9080 Melphalan flufenamide Pepaxto® 5/24/2021 5/24/2021 G2082 Esketamine Spravato® 2020 2020 (up to 56 mg plus observation) G2083 Esketamine Spravato® 2020 2020 (greater than 56 mg plus observation) 1 Medical Drug and Step Therapy Prior Authorization List for Medicare Plus BlueSM and BCN AdvantageSM members Revised September 2021 Prior authorization Submit authorization requirement effective date request through AIM HCPCS Step therapy Medicare BCN Specialty codes Generic name Trade name requirement Plus Blue Advantage NovoLogix® Health® J0129 Abatacept Orencia® 2017 2018 Try/fail Inflectra® or Renflexis®. These preferred drugs don’t require authorization. J0178 Aflibercept Eylea® 2017 2017 J0179 Brolucizumab-dbll Beovu® 2020 2020 J0180 Agalsidase beta Fabrazyme® 2017 2017 J0221 Alglucosidase alfa, 10mg Lumizyme® -
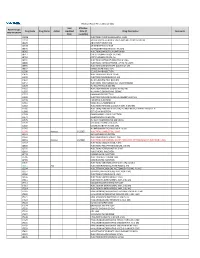
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Prior Authorization Cushing’S – Isturisa® (Osilodrostat Tablets)
Cigna National Formulary Coverage Policy Prior Authorization Cushing’s – Isturisa® (osilodrostat tablets) Table of Contents Product Identifier(s) National Formulary Medical Necessity ................ 1 64705 Conditions Not Covered....................................... 2 Background .......................................................... 2 References .......................................................... 3 Revision History ................................................... 3 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. -

Tables-Of-Phase-3-Mabs.Pdf
Table 3. Monoclonal antibodies in Phase 2/3 or 3 clinical studies for cancer indications Most advanced Primary sponsoring company INN or code name Molecular format Target(s) phase Phase 3 indications Therapeutic area Janssen Research & Development, LLC JNJ-56022473 Humanized mAb CD123 Phase 2/3 Acute myeloid leukemia Cancer Murine IgG1, Actinium Pharmaceuticals Iomab-B radiolabeled CD45 Phase 3 Acute myeloid leukemia Cancer Humanized IgG1, Seattle Genetics Vadastuximab talirine ADC CD33 Phase 3 Acute myeloid leukemia Cancer TG Therapeutics Ublituximab Chimeric IgG1 CD20 Phase 3 Chronic lymphocytic leukemia Cancer Xencor XMAB-5574, MOR208 Humanized IgG1 CD19 Phase 2/3 Diffuse large B-cell lymphoma Cancer Moxetumomab Murine IgG1 dsFv, AstraZeneca/MedImmune LLC pasudotox immunotoxin CD22 Phase 3 Hairy cell leukemia Cancer Humanized scFv, Viventia Bio Oportuzumab monatox immunotoxin EpCAM Phase 3 Bladder cancer Cancer scFv-targeted liposome containing Merrimack Pharmaceuticals MM-302 doxorubicin HER2 Phase 2/3 Breast cancer Cancer MacroGenics Margetuximab Chimeric IgG1 HER2 Phase 3 Breast cancer Cancer Gastric cancer or gastroesophageal junction Gilead Sciences GS-5745 Humanized IgG4 MMP9 Phase 3 adenocarcinoma; ulcerative colitis (Phase 2/3) Cancer; Immune-mediated disorders Depatuxizumab Humanized IgG1, AbbVie mafodotin ADC EGFR Phase 2/3 Glioblastoma Cancer AstraZeneca/MedImmune LLC Tremelimumab Human IgG2 CTLA4 Phase 3 NSCLC, head & neck cancer, bladder cancer Cancer NSCLC, head & neck cancer, bladder cancer, breast AstraZeneca/MedImmune -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities