Pharmacodynamic Evaluation of Suppression of in Vitro Resistance In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Items
Essential Medicines List (EML) 2019 Application for the inclusion of imipenem/cilastatin, meropenem and amoxicillin/clavulanic acid in the WHO Model List of Essential Medicines, as reserve second-line drugs for the treatment of multidrug-resistant tuberculosis (complementary lists of anti-tuberculosis drugs for use in adults and children) General items 1. Summary statement of the proposal for inclusion, change or deletion This application concerns the updating of the forthcoming WHO Model List of Essential Medicines (EML) and WHO Model List of Essential Medicines for Children (EMLc) to include the following medicines: 1) Imipenem/cilastatin (Imp-Cln) to the main list but NOT the children’s list (it is already mentioned on both lists as an option in section 6.2.1 Beta Lactam medicines) 2) Meropenem (Mpm) to both the main and the children’s lists (it is already on the list as treatment for meningitis in section 6.2.1 Beta Lactam medicines) 3) Clavulanic acid to both the main and the children’s lists (it is already listed as amoxicillin/clavulanic acid (Amx-Clv), the only commercially available preparation of clavulanic acid, in section 6.2.1 Beta Lactam medicines) This application makes reference to amendments recommended in particular to section 6.2.4 Antituberculosis medicines in the latest editions of both the main EML (20th list) and the EMLc (6th list) released in 2017 (1),(2). On the basis of the most recent Guideline Development Group advising WHO on the revision of its guidelines for the treatment of multidrug- or rifampicin-resistant (MDR/RR-TB)(3), the applicant considers that the three agents concerned be viewed as essential medicines for these forms of TB in countries. -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -
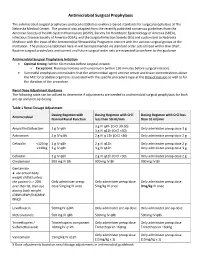
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

Management of Penicillin and Beta-Lactam Allergy
Management of Penicillin and Beta-Lactam Allergy (NB Provincial Health Authorities Anti-Infective Stewardship Committee, September 2017) Key Points • Beta-lactams are generally safe; allergic and adverse drug reactions are over diagnosed and over reported • Nonpruritic, nonurticarial rashes occur in up to 10% of patients receiving penicillins. These rashes are usually not allergic and are not a contraindication to the use of a different beta-lactam • The frequently cited risk of 8 to 10% cross-reactivity between penicillins and cephalosporins is an overestimate based on studies from the 1970’s that are now considered flawed • Expect new intolerances (i.e. any allergy or adverse reaction reported in a drug allergy field) to be reported after 0.5 to 4% of all antimicrobial courses depending on the gender and specific antimicrobial. Expect a higher incidence of new intolerances in patients with three or more prior medication intolerances1 • For type-1 immediate hypersensitivity reactions (IgE-mediated), cross-reactivity among penicillins (table 1) is expected due to similar core structure and/or major/minor antigenic determinants, use not recommended without desensitization • For type-1 immediate hypersensitivity reactions, cross-reactivity between penicillins (table 1) and cephalosporins is due to similarities in the side chains; risk of cross-reactivity will only be significant between penicillins and cephalosporins with similar side chains • Only type-1 immediate hypersensitivity to a penicillin manifesting as anaphylaxis, bronchospasm, -

Renal Fanconi Syndrome with Meropenem/Amoxicillin-Clavulanate During Treatment of Extensively Drug- Resistant Tuberculosis
AGORA | CORRESPONDENCE Renal Fanconi syndrome with meropenem/amoxicillin-clavulanate during treatment of extensively drug- resistant tuberculosis To the Editor: We read with interest the papers by TIBERI et al. [1, 2] describing the effectiveness of meropenem/ clavulanate in treating multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) patients. In these analyses, 96 patients were treated with meropenem/clavulanate for a median of 85 (interquartile range (IQR) 49–156) days with six adverse events, and 84 patients were treated with imipenem/clavulanate for a median (IQR) of 187 (60–428) days and three adverse events, none renally related. We report a patient with XDR-TB who developed renal Fanconi syndrome apparently due to meropenem/amoxicillin-clavulanate. A 25-year-old South Asian female with intermittently treated pulmonary XDR-TB since 2006, presented to the National Institutes of Health (NIH) in 2015 for treatment under an institutional review board-approved clinical protocol. She was started on daily linezolid, bedaquiline, clofazimine and meropenem 1.5 g i.v. with amoxicillin-clavulanate 500/125 mg orally, both every 8 h. Within 1 month, she − developed mild hypophosphataemia (1.8–2.0 mg·dL 1). Her renal function and urinalysis remained normal at 3 months. Her sputum cultures converted to negative after 98 days of treatment. At 4 months, − she developed a mild normal anion gap metabolic acidosis (bicarbonate 18–19 mmol·L 1). After 6–7 months, she developed fatigue, moderate proteinuria (600 mg/24 h), aminoaciduria, glycosuria (2–3+ with normal serum glucose), worsening hypophosphataemia with a high urine fractional excretion of phosphorus (24%) consistent with inappropriate urinary phosphate wasting, and hypouricaemia (uric acid − 0.9 mg·dL 1), in addition to the normal anion gap metabolic acidosis, all consistent with generalised proximal tubular dysfunction and renal Fanconi syndrome. -

Carbapenem-Resistant Enterobacteriaceae Investigative Guidelines December 2019
PUBLIC HEALTH DIVISION Acute and Communicable Disease Prevention Carbapenem-Resistant Enterobacteriaceae Investigative Guidelines December 2019 1. DISEASE REPORTING 1.1 Purpose of Reporting and Surveillance 1. To prevent transmission of infections with carbapenem-resistant Enterobacteriaceae (CRE) between patients, within or among health care facilities, or between health care facilities and the community. 2. To prevent CRE from becoming endemic in Oregon, necessitating empiric use of even broader- spectrum antibiotics. 3. To identify outbreaks and potential sources or sites of ongoing transmission. 4. To better characterize the epidemiology of these infections. 1.2 Laboratory and Physician Reporting Requirements 1. Providers and laboratories must report cases to local public health authorities (LPHAs) within one working day. 2. Clinical and reference laboratories must forward isolates from any sterile or non-sterile site (e.g., urine, blood, sputum, endotracheal aspirate, BAL, wound) that meet the confirmed CRE case definition below along with the automated test system susceptibility printouts (Vitek or Microscan report) to the Oregon State Public Health Laboratory (OSPHL). 3. Isolates of Proteus, Providencia, or Morganella which show only imipenem non-susceptibility, in the absence of resistance to another carbapenem, do not need to be submitted. 1.3 Local Public Health Authority Reporting and Follow-Up Responsibilities 1. LPHAs will confirm that a case meets the case definition by reviewing the isolate's susceptibility information (antibiogram) as necessary, or in consultation with the ACDP epidemiologist. Both minimum inhibitory concentration (MIC) values and interpretations are needed to verify a case meets the definition. (See Confirmed Case §3.1.) 2. If a case meets the case definition, the LPHA will investigate. -

Merrem I.V. for Intravenous Use Only
NDA 50-706/S-022 Page 3 MERREM® I.V. (meropenem for injection) FOR INTRAVENOUS USE ONLY To reduce the development of drug-resistant bacteria and maintain the effectiveness of MERREM® I.V. (meropenem for injection) and other antibacterial drugs, MERREM I.V. should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION MERREM® I.V. (meropenem for injection) is a sterile, pyrogen-free, synthetic, broad-spectrum, carbapenem antibiotic for intravenous administration. It is (4R,5S,6S)-3-[[(3S,5S)-5- (Dimethylcarbamoyl)-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1- azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid trihydrate. Its empirical formula is C17H25N3O5S•3H2O with a molecular weight of 437.52. Its structural formula is: MERREM I.V. is a white to pale yellow crystalline powder. The solution varies from colorless to yellow depending on the concentration. The pH of freshly constituted solutions is between 7.3 and 8.3. Meropenem is soluble in 5% monobasic potassium phosphate solution, sparingly soluble in water, very slightly soluble in hydrated ethanol, and practically insoluble in acetone or ether. When constituted as instructed (see DOSAGE AND ADMINISTRATION; PREPARATION OF SOLUTION), each 1 g MERREM I.V. vial will deliver 1 g of meropenem and 90.2 mg of sodium as sodium carbonate (3.92 mEq). Each 500 mg MERREM I.V. vial will deliver 500 mg meropenem and 45.1 mg of sodium as sodium carbonate (1.96 mEq). CLINICAL PHARMACOLOGY At the end of a 30-minute intravenous infusion of a single dose of MERREM I.V. -

Efficacy of BAL30072 in Experimental Respiratory Tract Infections UNT Health Science Center 49Th ICAAC Meeting 3500 Camp Bowie Blvd
Poster F1-1485 Contact Information: Efficacy of BAL30072 in Experimental Respiratory Tract Infections UnT Health Science Center 49th ICAAC Meeting 3500 Camp Bowie Blvd. Fort worth, TX 76107 1 1 1 1 1 2 San Francisco, CA W. J. WEISS *, M. E. PULSE , P. NGUYEN , J. PIERCE , J. SIMECKA , M. G. P. PAGE [email protected] 1 2 September 12-15, 2009 UNT Health Science Center, Ft. Worth, TX, Basilea Pharmaceutica Intl. Ltd, Basel, Switzerland www.hsc.unt.edu/preclinical Efficacy of BAL30072, Meropenem and Aztreonam following a single dose i.p. Efficacy of BAL30072, Meropenem and Imipenem following a single dose i.p. Chemical Structure of BAL30072 Abstract administration in the K. pneumoniae ATCC43816 lung infection administration in the P. aeruginosa ATCC39324 lung infection O Background: BAL30072 (BAL), a novel siderophore monobactam, exhibits potent activity against gram-negative non- fermentors. The current study was performed to determine the efficacy of BAL in murine lung infection models with Geomean Mean Log Geomean Mean Log OH isolates of K. pneumoniae (Kpn) and P. aeruginosa (Psa). Methods: Female CD-1 mice were infected intranasally with Compound Dose SD 10 Compound Dose SD 10 Kpn or Psa and treated IP with either single or multiple doses (5 – 100 mg/kg) of BAL, Aztreonam (Azt), Meropenem (Mero), Log 10 CFU reduction Log 10 CFU reduction Imipenem (Imi) or BAL:Mero (at a ratio of 1:1). Bacterial lung counts were determined at 24 hr following a single dose (+4 hrs post-infection) and survival curves were tracked following a multiple dosing regimen (bid x 3 days). -

Possible Factors Involved in Oral Inactivity of Meropenem, a Carbapenem Antibiotic
Pharmacology & Pharmacy, 2012, 3, 201-206 201 http://dx.doi.org/10.4236/pp.2012.32027 Published Online April 2012 (http://www.SciRP.org/journal/pp) Possible Factors Involved in Oral Inactivity of Meropenem, a Carbapenem Antibiotic Toshihide Saito, Rinako Sawazaki, Kaori Ujiie, Masako Oda, Hiroshi Saitoh* Department of Pharmaceutics, School of Pharmaceutical Sciences, Health Sciences University of Hokkaido, Ishikari-Tobetsu, Hok- kaido, Japan. Email: *[email protected] Received January 15th, 2012; revised February 12th, 2012; accepted March 5th, 2012 ABSTRACT Meropenem, a carbapenem antibiotic, is inactive after oral administration and administered exclusively by injection. In this study, in order to address the factors involved in the oral inactivity of meropenem, in vitro permeation characteris- tics across rat ileal segments was investigated using diffusion cells. Moreover, stability of meropenem was evaluated in the Japanese Pharmacopoeia (JP) 1st and 2nd fluid for disintegration test. Cefotaxime, ceftibuten, and faropenem were used for comparison. The permeation of meropenem across rat ileal segments was approximately 5-fold greater in sec- retory direction than in absorptive direction. The secretory-oriented transport of meropenem markedly diminished by replacement of D-glucose in the experimental medium with unmetabolizing 3-O-methyl-D-glucose, suggesting that the secretory transport of meropenem was an energy-dependent process. Cefotaxime exhibited extensively secretory-ori- ented permeation. On the other hand, much weaker directionalities were observed in ceftibuten and faropenem. While meropenem as well as other three β-lactam antibiotics was stable in JP 2nd fluid (pH 6.8), it declined rapidly in JP 1st fluid (pH 1.2). -

Efficacy of Ertapenem for Treatment of Bloodstream Infections Caused By
Efficacy of Ertapenem for Treatment of Bloodstream Infections Caused by Extended-Spectrum--Lactamase-Producing Enterobacteriaceae Vicki L. Collins,a Dror Marchaim,a Jason M. Pogue,b Judy Moshos,a Suchitha Bheemreddy,a Bharath Sunkara,a Alex Shallal,a Neelu Chugh,a Sara Eiseler,a Pragati Bhargava,a Christopher Blunden,a Paul R. Lephart,c Babar Irfan Memon,a Kayoko Hayakawa,a Odaliz Abreu-Lanfranco,a Teena Chopra,a L. Silvia Munoz-Price,d Yehuda Carmeli,e and Keith S. Kayea Division of Infectious Diseases,a Department of Pharmacy Services,b and Department of Clinical Microbiology,c Detroit Medical Center, Wayne State University, Detroit, Michigan, USA; Division of Infectious Diseases, Department of Medicine and Department of Public Health and Epidemiology, University of Miami, Miami, Florida, USAd; and Division of Epidemiology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israele Ertapenem is active against extended-spectrum--lactamase (ESBL)-producing Enterobacteriaceae organisms but inactive against Pseudomonas aeruginosa and Acinetobacter baumannii. Due to a lack of therapeutic data for ertapenem in the treatment of ESBL bloodstream infections (BSIs), group 2 carbapenems (e.g., imipenem or meropenem) are often preferred for treatment of ESBL-producing Enterobacteriaceae, although their antipseudomonal activity is unnecessary. From 2005 to 2010, 261 patients with ESBL BSIs were analyzed. Outcomes were equivalent between patients treated with ertapenem and those treated with group .(0.18 ؍ carbapenems (mortality rates of 6% and 18%, respectively; P 2 xtended-spectrum--lactamase (ESBL)-producing Entero- stay (LOS), deterioration in functional status (18), discharge to Ebacteriaceae organisms are recognized as an imminent threat a long-term care facility (LTCF) after being admitted from to public health (7, 26). -

Antibiotics Against Multidrug-Resistant Gram-Negative Bacteria
Journal of Clinical Medicine Review An Update on Eight “New” Antibiotics against Multidrug-Resistant Gram-Negative Bacteria Erlangga Yusuf * , Hannelore I. Bax, Nelianne J. Verkaik and Mireille van Westreenen Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, 3015 GD Rotterdam, The Netherlands; [email protected] (H.I.B.); [email protected] (N.J.V.); [email protected] (M.v.W.) * Correspondence: [email protected] Abstract: Infections in the ICU are often caused by Gram-negative bacteria. When these microorgan- isms are resistant to third-generation cephalosporines (due to extended-spectrum (ESBL) or AmpC beta-lactamases) or to carbapenems (for example carbapenem producing Enterobacteriales (CPE)), the treatment options become limited. In the last six years, fortunately, there have been new an- tibiotics approved by the U.S. Food and Drug Administration (FDA) with predominant activities against Gram-negative bacteria. We aimed to review these antibiotics: plazomicin, eravacycline, temocillin, cefiderocol, ceftazidime/avibactam, ceftolozane/tazobactam, meropenem/vaborbactam, and imipenem/relebactam. Temocillin is an antibiotic that was only approved in Belgium and the UK several decades ago. We reviewed the in vitro activities of these new antibiotics, especially against ESBL and CPE microorganisms, potential side effects, and clinical studies in complicated urinary tract infections (cUTI), intra-abdominal infections (cIAI), and hospital-acquired pneumonia/ventilator- associatedpneumonia (HAP/VAP). All of these new antibiotics are active against ESBL, and almost all of them are active against CPE caused by KPC beta-lactamase, but only some of them are active against CPE due to MBL or OXA beta-lactamases.