Possible Factors Involved in Oral Inactivity of Meropenem, a Carbapenem Antibiotic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacodynamic Evaluation of Suppression of in Vitro Resistance In
www.nature.com/scientificreports OPEN Pharmacodynamic evaluation of suppression of in vitro resistance in Acinetobacter baumannii strains using polymyxin B‑based combination therapy Nayara Helisandra Fedrigo1, Danielle Rosani Shinohara1, Josmar Mazucheli2, Sheila Alexandra Belini Nishiyama1, Floristher Elaine Carrara‑Marroni3, Frederico Severino Martins4, Peijuan Zhu5, Mingming Yu6, Sherwin Kenneth B. Sy2 & Maria Cristina Bronharo Tognim1* The emergence of polymyxin resistance in Gram‑negative bacteria infections has motivated the use of combination therapy. This study determined the mutant selection window (MSW) of polymyxin B alone and in combination with meropenem and fosfomycin against A. baumannii strains belonging to clonal lineages I and III. To evaluate the inhibition of in vitro drug resistance, we investigate the MSW‑derived pharmacodynamic indices associated with resistance to polymyxin B administrated regimens as monotherapy and combination therapy, such as the percentage of each dosage interval that free plasma concentration was within the MSW (%TMSW) and the percentage of each dosage interval that free plasma concentration exceeded the mutant prevention concentration (%T>MPC). The MSW of polymyxin B varied between 1 and 16 µg/mL for polymyxin B‑susceptible strains. The triple combination of polymyxin B with meropenem and fosfomycin inhibited the polymyxin B‑resistant subpopulation in meropenem‑resistant isolates and polymyxin B plus meropenem as a double combination sufciently inhibited meropenem‑intermediate, and susceptible strains. T>MPC 90% was reached for polymyxin B in these combinations, while %TMSW was 0 against all strains. TMSW for meropenem and fosfomycin were also reduced. Efective antimicrobial combinations signifcantly reduced MSW. The MSW‑derived pharmacodynamic indices can be used for the selection of efective combination regimen to combat the polymyxin B‑resistant strain. -

General Items
Essential Medicines List (EML) 2019 Application for the inclusion of imipenem/cilastatin, meropenem and amoxicillin/clavulanic acid in the WHO Model List of Essential Medicines, as reserve second-line drugs for the treatment of multidrug-resistant tuberculosis (complementary lists of anti-tuberculosis drugs for use in adults and children) General items 1. Summary statement of the proposal for inclusion, change or deletion This application concerns the updating of the forthcoming WHO Model List of Essential Medicines (EML) and WHO Model List of Essential Medicines for Children (EMLc) to include the following medicines: 1) Imipenem/cilastatin (Imp-Cln) to the main list but NOT the children’s list (it is already mentioned on both lists as an option in section 6.2.1 Beta Lactam medicines) 2) Meropenem (Mpm) to both the main and the children’s lists (it is already on the list as treatment for meningitis in section 6.2.1 Beta Lactam medicines) 3) Clavulanic acid to both the main and the children’s lists (it is already listed as amoxicillin/clavulanic acid (Amx-Clv), the only commercially available preparation of clavulanic acid, in section 6.2.1 Beta Lactam medicines) This application makes reference to amendments recommended in particular to section 6.2.4 Antituberculosis medicines in the latest editions of both the main EML (20th list) and the EMLc (6th list) released in 2017 (1),(2). On the basis of the most recent Guideline Development Group advising WHO on the revision of its guidelines for the treatment of multidrug- or rifampicin-resistant (MDR/RR-TB)(3), the applicant considers that the three agents concerned be viewed as essential medicines for these forms of TB in countries. -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -
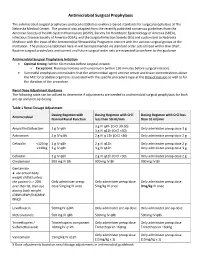
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment. -

Dose Amoxicillin/Clavulanate
1 Clinical trial of the treatment of acute sinusitis with standard-dose vs. high- dose amoxicillin/clavulanate Principal Investigator: Paul Sorum, MD, PhD, Professor of Internal Medicine and Pediatrics Co-Investigators: Andrea Matho, MD, 2nd year Med-Ped resident (the four 2nd-year residents will be responsible for the timely follow-up of enrolled patients) Mary Mulqueen, MD, 2nd year Med-Ped resident Aaron Quidort, MD, 2nd year Med-Ped resident Miyuki Tanino, MD, 2nd year Med-Ped resident Sujata Kane, PA-C (who will be enrolling the most patients) Christine McGovern, MS, PA-C (who will work with the residents to assure the smooth enrollment and complete follow-up of patients) Joseph Wayne, MD, MPH, Associate Professor of Internal Medicine and Pediatrics Gina Garrison, Pharm D, Associate Professor of Pharmacy Practice, Albany College of Pharmacy and Health Sciences (who will prepare the medications) Sub-Investigators The other attending physicians at the AMC Internal Medicine and Pediatrics office (who will be enrolling patients): Rahim Dhanani, MD Elizabeth Higgins, MD Hamish Kerr, MD Deborah Light, MD Jennifer Lindstrom, MD Tricia Pelnik-Fecko, MD Ivelisse Verrico, MD Kathleen Zabinski-Kramer, MD The other Medicine-Pediatrics residents (who will be enrolling patients and making follow-up telephone calls) Brady Bowen, DO Melanie Ecung Deyss, MD Laura El-Hage, MD Sara Khalil, MD Preetha Kurian, MD Daniel Lavelle, MD Manpreet Mann, MD Lyndsay Molinari, MD Uwa Otabor, MD Samuel Park, MD Britta Sundquist, MD Lisa Thaler, DO 2 Others Nursing staff (who will provide the patients with documents relating to the study) Medical students for research experience or work-study (who will help the residents to follow-up telephone calls and who will, if possible, enter data into the Excel data base) (to be set up) Statistical consultant: Michael Mulvihill, DPH, Einstein Medical Center, NYC A. -

Consideration of Antibacterial Medicines As Part Of
Consideration of antibacterial medicines as part of the revisions to 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) Section 6.2 Antibacterials including Access, Watch and Reserve Lists of antibiotics This summary has been prepared by the Health Technologies and Pharmaceuticals (HTP) programme at the WHO Regional Office for Europe. It is intended to communicate changes to the 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) to national counterparts involved in the evidence-based selection of medicines for inclusion in national essential medicines lists (NEMLs), lists of medicines for inclusion in reimbursement programs, and medicine formularies for use in primary, secondary and tertiary care. This document does not replace the full report of the WHO Expert Committee on Selection and Use of Essential Medicines (see The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2019 (WHO Technical Report Series, No. 1021). Licence: CC BY-NC-SA 3.0 IGO: https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1) and Corrigenda (March 2020) – TRS1021 (https://www.who.int/medicines/publications/essentialmedicines/TRS1021_corrigenda_March2020. pdf?ua=1). Executive summary of the report: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO- MVP-EMP-IAU-2019.05-eng.pdf?ua=1. -

WO 2010/025328 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 4 March 2010 (04.03.2010) WO 2010/025328 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US2009/055306 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 28 August 2009 (28.08.2009) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/092,497 28 August 2008 (28.08.2008) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): FOR¬ GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, EST LABORATORIES HOLDINGS LIMITED [IE/ ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, —]; 18 Parliament Street, Milner House, Hamilton, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, Bermuda HM12 (BM). -

Management of Penicillin and Beta-Lactam Allergy
Management of Penicillin and Beta-Lactam Allergy (NB Provincial Health Authorities Anti-Infective Stewardship Committee, September 2017) Key Points • Beta-lactams are generally safe; allergic and adverse drug reactions are over diagnosed and over reported • Nonpruritic, nonurticarial rashes occur in up to 10% of patients receiving penicillins. These rashes are usually not allergic and are not a contraindication to the use of a different beta-lactam • The frequently cited risk of 8 to 10% cross-reactivity between penicillins and cephalosporins is an overestimate based on studies from the 1970’s that are now considered flawed • Expect new intolerances (i.e. any allergy or adverse reaction reported in a drug allergy field) to be reported after 0.5 to 4% of all antimicrobial courses depending on the gender and specific antimicrobial. Expect a higher incidence of new intolerances in patients with three or more prior medication intolerances1 • For type-1 immediate hypersensitivity reactions (IgE-mediated), cross-reactivity among penicillins (table 1) is expected due to similar core structure and/or major/minor antigenic determinants, use not recommended without desensitization • For type-1 immediate hypersensitivity reactions, cross-reactivity between penicillins (table 1) and cephalosporins is due to similarities in the side chains; risk of cross-reactivity will only be significant between penicillins and cephalosporins with similar side chains • Only type-1 immediate hypersensitivity to a penicillin manifesting as anaphylaxis, bronchospasm, -

Renal Fanconi Syndrome with Meropenem/Amoxicillin-Clavulanate During Treatment of Extensively Drug- Resistant Tuberculosis
AGORA | CORRESPONDENCE Renal Fanconi syndrome with meropenem/amoxicillin-clavulanate during treatment of extensively drug- resistant tuberculosis To the Editor: We read with interest the papers by TIBERI et al. [1, 2] describing the effectiveness of meropenem/ clavulanate in treating multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) patients. In these analyses, 96 patients were treated with meropenem/clavulanate for a median of 85 (interquartile range (IQR) 49–156) days with six adverse events, and 84 patients were treated with imipenem/clavulanate for a median (IQR) of 187 (60–428) days and three adverse events, none renally related. We report a patient with XDR-TB who developed renal Fanconi syndrome apparently due to meropenem/amoxicillin-clavulanate. A 25-year-old South Asian female with intermittently treated pulmonary XDR-TB since 2006, presented to the National Institutes of Health (NIH) in 2015 for treatment under an institutional review board-approved clinical protocol. She was started on daily linezolid, bedaquiline, clofazimine and meropenem 1.5 g i.v. with amoxicillin-clavulanate 500/125 mg orally, both every 8 h. Within 1 month, she − developed mild hypophosphataemia (1.8–2.0 mg·dL 1). Her renal function and urinalysis remained normal at 3 months. Her sputum cultures converted to negative after 98 days of treatment. At 4 months, − she developed a mild normal anion gap metabolic acidosis (bicarbonate 18–19 mmol·L 1). After 6–7 months, she developed fatigue, moderate proteinuria (600 mg/24 h), aminoaciduria, glycosuria (2–3+ with normal serum glucose), worsening hypophosphataemia with a high urine fractional excretion of phosphorus (24%) consistent with inappropriate urinary phosphate wasting, and hypouricaemia (uric acid − 0.9 mg·dL 1), in addition to the normal anion gap metabolic acidosis, all consistent with generalised proximal tubular dysfunction and renal Fanconi syndrome. -

Treatment of Drug-Resistant Tuberculosis an Official ATS/CDC/ERS/IDSA Clinical Practice Guideline Payam Nahid, Sundari R
AMERICAN THORACIC SOCIETY DOCUMENTS Treatment of Drug-Resistant Tuberculosis An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline Payam Nahid, Sundari R. Mase, Giovanni Battista Migliori, Giovanni Sotgiu, Graham H. Bothamley, Jan L. Brozek, Adithya Cattamanchi, J. Peter Cegielski, Lisa Chen, Charles L. Daley, Tracy L. Dalton, Raquel Duarte, Federica Fregonese, C. Robert Horsburgh, Jr., Faiz Ahmad Khan, Fayez Kheir, Zhiyi Lan, Alfred Lardizabal, Michael Lauzardo, Joan M. Mangan, Suzanne M. Marks, Lindsay McKenna, Dick Menzies, Carole D. Mitnick, Diana M. Nilsen, Farah Parvez, Charles A. Peloquin, Ann Raftery, H. Simon Schaaf, Neha S. Shah, Jeffrey R. Starke, John W. Wilson, Jonathan M. Wortham, Terence Chorba, and Barbara Seaworth; on behalf of the American Thoracic Society, U.S. Centers for Disease Control and Prevention, European Respiratory Society, and Infectious Diseases Society of America THIS OFFICIAL CLINICAL PRACTICE GUIDELINE WAS APPROVED BY THE AMERICAN THORACIC SOCIETY, THE EUROPEAN RESPIRATORY SOCIETY, AND THE INFECTIOUS DISEASES SOCIETY OF AMERICA SEPTEMBER 2019, AND WAS CLEARED BY THE U.S. CENTERS FOR DISEASE CONTROL AND PREVENTION SEPTEMBER 2019 Background: The American Thoracic Society, U.S. Centers for was judged to be very low, because the data came Disease Control and Prevention, European Respiratory Society, and from observational studies with significant loss to follow-up Infectious Diseases Society of America jointly sponsored this new and imbalance in background regimens between comparator practice guideline on the treatment of drug-resistant tuberculosis groups. Good practices in the management of MDR-TB are (DR-TB). The document includes recommendations on the described. On the basis of the evidence review, a clinical strategy treatment of multidrug-resistant TB (MDR-TB) as well as tool for building a treatment regimen for MDR-TB is also isoniazid-resistant but rifampin-susceptible TB. -

Comparison of Effectiveness and Safety of Imipenem/Clavulanate- Versus Meropenem/Clavulanate-Containing Regimens in the Treatment of MDR- and XDR-TB
ORIGINAL ARTICLE TUBERCULOSIS Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB Simon Tiberi1,30, Giovanni Sotgiu2,30, Lia D’Ambrosio3,4,30, Rosella Centis3,30, Marcos Abdo Arbex5,6, Edith Alarcon Arrascue7,8, Jan Willem Alffenaar9, Jose A. Caminero8,10, Mina Gaga11, Gina Gualano12, Alena Skrahina13, Ivan Solovic14, Giorgia Sulis15, Marina Tadolini16, Valentina Alarcon Guizado17, Saverio De Lorenzo18, Aurora Jazmín Roby Arias19, Anna Scardigli8, Onno W. Akkerman20, Alena Aleksa21, Janina Artsukevich21, Vera Auchynka13, Eduardo Henrique Bonini5,6, Félix Antonio Chong Marín19, Lorena Collahuazo López19, Gerard de Vries22, Simone Dore2, Heinke Kunst23, Alberto Matteelli15, Charalampos Moschos11, Fabrizio Palmieri12, Apostolos Papavasileiou11, Marie-Christine Payen24, Andrea Piana2, Antonio Spanevello25,26, Dante Vargas Vasquez27, Pietro Viggiani18, Veronica White28, Alimuddin Zumla29 and Giovanni Battista Migliori3 ABSTRACT No large study to date has ever evaluated the effectiveness, safety and tolerability of imipenem/clavulanate versus meropenem/clavulanate to treat multidrug- and extensively drug-resistant tuberculosis (MDR- and XDR-TB). The aim of this observational study was to compare the therapeutic contribution of imipenem/clavulanate versus meropenem/clavulanate added to background regimens to treat MDR- and XDR-TB cases. 84 patients treated with imipenem/clavulanate-containing regimens showed a similar median number of antibiotic resistances (8 versus 8) but more fluoroquinolone resistance (79.0% versus 48.9%, p<0.0001) and higher XDR-TB prevalence (67.9% versus 49.0%, p=0.01) in comparison with 96 patients exposed to meropenem/clavulanate-containing regimens. Patients were treated with imipenem/clavulanate- and meropenem/clavulanate-containing regimens for a median (interquartile range) of 187 (60–428) versus 85 (49–156) days, respectively.