Severe Sepsis and Septic Shock Antibiotic Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ceftazidime for Injection) PHARMACY BULK PACKAGE – NOT for DIRECT INFUSION
PRESCRIBING INFORMATION FORTAZ® (ceftazidime for injection) PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of FORTAZ and other antibacterial drugs, FORTAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibacterial drug for parenteral administration. It is the pentahydrate of pyridinium, 1-[[7-[[(2-amino-4 thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1 azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]. It has the following structure: The molecular formula is C22H32N6O12S2, representing a molecular weight of 636.6. FORTAZ is a sterile, dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The sodium carbonate at a concentration of 118 mg/g of ceftazidime activity has been admixed to facilitate dissolution. The total sodium content of the mixture is approximately 54 mg (2.3 mEq)/g of ceftazidime activity. The Pharmacy Bulk Package vial contains 709 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3mEq) per gram of ceftazidime. FORTAZ in sterile crystalline form is supplied in Pharmacy Bulk Packages equivalent to 6g of anhydrous ceftazidime. The Pharmacy Bulk Package bottle is a container of sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous use. THE PHARMACY BULK PACKAGE IS NOT FOR DIRECT INFUSION, FURTHER DILUTION IS REQUIRED BEFORE USE. -

Cefoxitin Versus Piperacillin– Tazobactam As Surgical Antibiotic Prophylaxis in Patients Undergoing Pancreatoduodenectomy: Protocol for a Randomised Controlled Trial
Open access Protocol BMJ Open: first published as 10.1136/bmjopen-2020-048398 on 4 March 2021. Downloaded from Cefoxitin versus piperacillin– tazobactam as surgical antibiotic prophylaxis in patients undergoing pancreatoduodenectomy: protocol for a randomised controlled trial Nicole M Nevarez ,1 Brian C Brajcich,2 Jason Liu,2,3 Ryan Ellis,2 Clifford Y Ko,2 Henry A Pitt,4 Michael I D'Angelica,5 Adam C Yopp1 To cite: Nevarez NM, ABSTRACT Strengths and limitations of this study Brajcich BC, Liu J, et al. Introduction Although antibiotic prophylaxis is Cefoxitin versus piperacillin– established in reducing postoperative surgical site tazobactam as surgical ► A major strength of this study is the multi- infections (SSIs), the optimal antibiotic for prophylaxis in antibiotic prophylaxis institutional, double- arm, randomised controlled in patients undergoing pancreatoduodenectomy (PD) remains unclear. The study trial design. objective is to evaluate if administration of piperacillin– pancreatoduodenectomy: ► A limitation of this study is that all perioperative care protocol for a randomised tazobactam as antibiotic prophylaxis results in decreased is at the discretion of the operating surgeon and is controlled trial. BMJ Open 30- day SSI rate compared with cefoxitin in patients not standardised. 2021;11:e048398. doi:10.1136/ undergoing elective PD. ► All data will be collected through the American bmjopen-2020-048398 Methods and analysis This study will be a multi- College of Surgeons National Surgical Quality ► Prepublication history for institution, double- arm, non- blinded randomised controlled Improvement Program, which is a strength for its this paper is available online. superiority trial. Adults ≥18 years consented to undergo PD ease of use but a limitation due to the variety of data To view these files, please visit for all indications who present to institutions participating included. -

Pharmacodynamic Evaluation of Suppression of in Vitro Resistance In
www.nature.com/scientificreports OPEN Pharmacodynamic evaluation of suppression of in vitro resistance in Acinetobacter baumannii strains using polymyxin B‑based combination therapy Nayara Helisandra Fedrigo1, Danielle Rosani Shinohara1, Josmar Mazucheli2, Sheila Alexandra Belini Nishiyama1, Floristher Elaine Carrara‑Marroni3, Frederico Severino Martins4, Peijuan Zhu5, Mingming Yu6, Sherwin Kenneth B. Sy2 & Maria Cristina Bronharo Tognim1* The emergence of polymyxin resistance in Gram‑negative bacteria infections has motivated the use of combination therapy. This study determined the mutant selection window (MSW) of polymyxin B alone and in combination with meropenem and fosfomycin against A. baumannii strains belonging to clonal lineages I and III. To evaluate the inhibition of in vitro drug resistance, we investigate the MSW‑derived pharmacodynamic indices associated with resistance to polymyxin B administrated regimens as monotherapy and combination therapy, such as the percentage of each dosage interval that free plasma concentration was within the MSW (%TMSW) and the percentage of each dosage interval that free plasma concentration exceeded the mutant prevention concentration (%T>MPC). The MSW of polymyxin B varied between 1 and 16 µg/mL for polymyxin B‑susceptible strains. The triple combination of polymyxin B with meropenem and fosfomycin inhibited the polymyxin B‑resistant subpopulation in meropenem‑resistant isolates and polymyxin B plus meropenem as a double combination sufciently inhibited meropenem‑intermediate, and susceptible strains. T>MPC 90% was reached for polymyxin B in these combinations, while %TMSW was 0 against all strains. TMSW for meropenem and fosfomycin were also reduced. Efective antimicrobial combinations signifcantly reduced MSW. The MSW‑derived pharmacodynamic indices can be used for the selection of efective combination regimen to combat the polymyxin B‑resistant strain. -

General Items
Essential Medicines List (EML) 2019 Application for the inclusion of imipenem/cilastatin, meropenem and amoxicillin/clavulanic acid in the WHO Model List of Essential Medicines, as reserve second-line drugs for the treatment of multidrug-resistant tuberculosis (complementary lists of anti-tuberculosis drugs for use in adults and children) General items 1. Summary statement of the proposal for inclusion, change or deletion This application concerns the updating of the forthcoming WHO Model List of Essential Medicines (EML) and WHO Model List of Essential Medicines for Children (EMLc) to include the following medicines: 1) Imipenem/cilastatin (Imp-Cln) to the main list but NOT the children’s list (it is already mentioned on both lists as an option in section 6.2.1 Beta Lactam medicines) 2) Meropenem (Mpm) to both the main and the children’s lists (it is already on the list as treatment for meningitis in section 6.2.1 Beta Lactam medicines) 3) Clavulanic acid to both the main and the children’s lists (it is already listed as amoxicillin/clavulanic acid (Amx-Clv), the only commercially available preparation of clavulanic acid, in section 6.2.1 Beta Lactam medicines) This application makes reference to amendments recommended in particular to section 6.2.4 Antituberculosis medicines in the latest editions of both the main EML (20th list) and the EMLc (6th list) released in 2017 (1),(2). On the basis of the most recent Guideline Development Group advising WHO on the revision of its guidelines for the treatment of multidrug- or rifampicin-resistant (MDR/RR-TB)(3), the applicant considers that the three agents concerned be viewed as essential medicines for these forms of TB in countries. -
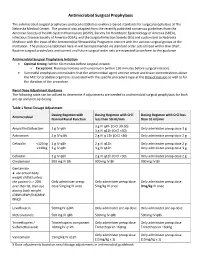
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Septic Shock V9.0 Patient Flow Map
Septic Shock v9.0 Patient Flow Map Approval & Citation Summary of Version Changes Explanation of Evidence Ratings Patient presents to the ED with fever and/or concern for infection and ED sepsis score ≥ 6 ! BPA fires Use the ED Suspected Septic Shock RN and Well-appearing patients should be placed pathway for all ill on the appropriate ED CSW pathway for Provider appearing patients their underlying condition (e.g. ED No including HemOnc/BMT, Huddle: HemOnc BMT Suspected Infection, ED Central Line Infection Is the patient ill Suspected Central Line Infection, ED and Neonates appearing? Neonatal Fever) Yes ED Septic Shock Pathway • Use ED Suspected Septic Shock Plan • Antibiotics and blood cultures for specific populations included Inpatient Admit Criteria Does NOT meet Inpatient Admit Minute criteria • Resolution of hypotension and no • Admit to ICU ongoing signs of sepsis after ≤ 40 ml / 60 Huddle: YES NO • Follow ICU Septic Shock Pathway kg NS bolus Does patient meet • Use PICU/CICU Septic Shock Admit • First dose antibiotics administered Inpatient admit Plan • RISK to follow criteria? • Antibiotics, blood cultures for specific populations included in sub plans Previously healthy > 30 days RISK RN to follow all • Admit to General Medicine patients admitted with • Follow Admit from ED Septic Shock concern for sepsis Pathway • Use Inpatient Septic Shock Plan ! Concern for evolving sepsis Previously healthy < 30 days Any • Admit to General Medicine admitted • Call RRT or Code Blue • Follow Neonatal Fever Pathway patient with • Follow Inpatient -

Evaluation and Management of the Polytraumatized Patient in Various Centers
World J. Surg. 7, 143-148, 1983 Wor Journal of Stirgery Evaluation and Management of the Polytraumatized Patient in Various Centers S. Olerud, M.D., and M. Allg6wer, M.D. The Akademiska Sjukhuset Uppsala, Sweden, and the Department of Surgery, Kantonsspital, Basel, Switzerland A questionnaire was sent to the following 6 trauma centers: Paris: Two or more peripheral, visceral, or com- University Hospital for Accident Surgery, Hannover, Fed- plex injuries with respiratory and circulatory fail- eral Republic of Germany (Prof. H. Tscherne); University ure. (This excludes patients who only have sus- of Munich, Department of Surgery, Klinikum Grossha- tained fractures.) dern, Munich, Federal Republic of Germany (Prof. G. Dallas: Multiply injured patient presenting le- Heberer); Akademiska Sjukhuset Uppsala, Sweden (Prof. sions to 2 cavities, associated with 2 or more long S. Olerud); University Hospital, Department of Surgery, bone failures; lesions to 1 cavity associated with 2 Basel, Switzerland (Prof. M. Allgiiwer); H6pital de la Piti~, or more long bone failures; or lesions to multiple Paris, France (Prof. R. Roy-Camille); and University of extremities (at minimum, 3 long bone failures). Texas Southwestern Medical School, Dallas, Texas, U.S.A. (Prof. B. Claudi). Their answers have been summarized in a few short paragraphs where tabulation was not possible, Do You Grade Polytrauma, and If So, How? and then mainly in tabular form for convenient comparison among the various centers. There seems to be considerable international agreement on the main points of early aggres- Hannover: Yes, with our own grading system along sive cardiopulmonary management to prevent multiple with ISS and AIS. -

Antimicrobial Stewardship Guidance
Antimicrobial Stewardship Guidance Federal Bureau of Prisons Clinical Practice Guidelines March 2013 Clinical guidelines are made available to the public for informational purposes only. The Federal Bureau of Prisons (BOP) does not warrant these guidelines for any other purpose, and assumes no responsibility for any injury or damage resulting from the reliance thereof. Proper medical practice necessitates that all cases are evaluated on an individual basis and that treatment decisions are patient-specific. Consult the BOP Clinical Practice Guidelines Web page to determine the date of the most recent update to this document: http://www.bop.gov/news/medresources.jsp Federal Bureau of Prisons Antimicrobial Stewardship Guidance Clinical Practice Guidelines March 2013 Table of Contents 1. Purpose ............................................................................................................................................. 3 2. Introduction ...................................................................................................................................... 3 3. Antimicrobial Stewardship in the BOP............................................................................................ 4 4. General Guidance for Diagnosis and Identifying Infection ............................................................. 5 Diagnosis of Specific Infections ........................................................................................................ 6 Upper Respiratory Infections (not otherwise specified) .............................................................................. -

Below Are the CLSI Breakpoints for Selected Bacteria. Please Use Your Clinical Judgement When Assessing Breakpoints
Below are the CLSI breakpoints for selected bacteria. Please use your clinical judgement when assessing breakpoints. The lowest number does NOT equal most potent antimicrobial. Contact Antimicrobial Stewardship for drug selection and dosing questions. Table 1: 2014 MIC Interpretive Standards for Enterobacteriaceae (includes E.coli, Klebsiella, Enterobacter, Citrobacter, Serratia and Proteus spp) Antimicrobial Agent MIC Interpretive Criteria (g/mL) Enterobacteriaceae S I R Ampicillin ≤ 8 16 ≥ 32 Ampicillin-sulbactam ≤ 8/4 16/8 ≥ 32/16 Aztreonam ≤ 4 8 ≥ 16 Cefazolin (blood) ≤ 2 4 ≥ 8 Cefazolin** (uncomplicated UTI only) ≤ 16 ≥ 32 Cefepime* ≤ 2 4-8* ≥ 16 Cefotetan ≤ 16 32 ≥ 64 Ceftaroline ≤ 0.5 1 ≥ 2 Ceftazidime ≤ 4 8 ≥ 16 Ceftriaxone ≤ 1 2 ≥ 4 Cefpodoxime ≤ 2 4 ≥ 8 Ciprofloxacin ≤ 1 2 ≥ 4 Ertapenem ≤ 0.5 1 ≥ 2 Fosfomycin ≤ 64 128 ≥256 Gentamicin ≤ 4 8 ≥ 16 Imipenem ≤ 1 2 ≥ 4 Levofloxacin ≤ 2 4 ≥ 8 Meropenem ≤ 1 2 ≥ 4 Piperacillin-tazobactam ≤ 16/4 32/4 – 64/4 ≥ 128/4 Trimethoprim-sulfamethoxazole ≤ 2/38 --- ≥ 4/76 *Susceptibile dose-dependent – see chart below **Cefazolin can predict results for cefaclor, cefdinir, cefpodoxime, cefprozil, cefuroxime axetil, cephalexin and loracarbef for uncomplicated UTIs due to E.coli, K.pneumoniae, and P.mirabilis. Cefpodoxime, cefinidir, and cefuroxime axetil may be tested individually because some isolated may be susceptible to these agents while testing resistant to cefazolin. Cefepime dosing for Enterobacteriaceae ( E.coli, Klebsiella, Enterobacter, Citrobacter, Serratia & Proteus spp) Susceptible Susceptible –dose-dependent (SDD) Resistant MIC </= 2 4 8 >/= 16 Based on dose of: 1g q12h 1g every 8h or 2g every 8 h Do not give 2g q12 Total dose 2g 3-4g 6g NA Table 2: 2014 MIC Interpretive Standards for Pseudomonas aeruginosa and Acinetobacter spp. -

Shock and Hemodynamic Monitoring
Shock and Hemodynamic Monitoring Matthew Bank, MD, FACS Assistant Professor Hofstra North Shore‐LIJ School of Medicine Director, Surgical Intensive Care Unit North Shore University Hospital I do not have any financial conflicts of interest to disclose for this presentation Shock • Multiple different strategies for classifying shock, but all forms of shock result in impaired oxygen delivery secondary to either one or both: – reduced cardiac output (cardiogenic, septic) OR – loss of effective intravascular volume (hypovolemic, neurogenic, anaphylactic, septic). Septic Shock –Gram Negative • Gram negative septic shock: —Very studied well studied in animal models —Lipopolysaccharide (LPS) in bacterial cell wall binds to LPS binding protein. —LPS‐LBP complex then binds to cell surface CD14 receptors on monocytes and macrophages. —The LPS‐LBP‐CD14 complex then activates cells via Toll‐like receptor‐4 (TLR4). —TLR4 then “activates” cells which produce a cytokine “cascade” of proinflamatory mediators. Septic Shock –Gram Negative • Tumor Necrosis Factor (TNF) – First cytokine produced in response to gram negative sepsis – Principal mediator for acute response to gram negative bacteria – Major source of TNF is from activated macrophages – High levels of TNF predict mortality and can cause apoptosis. Septic Shock –Gram Negative • Interleukin‐1 (IL‐1) – Levels of IL‐1 increase soon after TNF production in gram negative sepsis (second cytokine to be elevated) – IL‐1 produced by macrophages, neutrophils and endothelial cells – IL‐1 increases levels of next proinflammatory cytokines in cascade, IL‐2 and IL‐12. – IL‐1 does NOT cause apoptosis Septic Shock –Gram Negative • Interleukin‐10 – Anti‐inflammatory cytokine – Inhibits production of IL‐12 – Inhibits T‐cell activation Septic Shock –Gram Positive • Gram positive sepsis – Gram positive cell wall components are also known to be involved in septic response – Peptidoglycans – Teichoic Acid – Likely act in a similar manner as LPS, but less potent on a weight bases. -

Synermox 500 Mg/125 Mg Tablets
New Zealand Data Sheet 1. PRODUCT NAME Synermox 500 mg/125 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Synermox 500 mg/125 mg Tablets: Each film‐coated tablet contains amoxicillin trihydrate equivalent to 500 mg amoxicillin, with potassium clavulanate equivalent to 125 mg clavulanic acid. Excipient(s) with known effect For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Synermox 500 mg/125 mg Tablets: White to off‐white, oval shaped film‐coated tablets, debossed with “RX713” on one side and plain on the other. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Synermox is indicated in adults and children (see sections 4.2, 4.4 and 5.1) for short term treatment of common bacterial infections such as: Upper Respiratory Tract Infections (including ENT) e.g. Tonsillitis, sinusitis, otitis media. Lower Respiratory Tract Infection e.g. acute exacerbations of chronic bronchitis, lobar and broncho‐pneumonia. Genito‐urinary Tract Infections e.g. Cystitis, urethritis, pyelonephritis, female genital infections. Skin and Soft Tissue Infections. Bone and Joint Infections e.g. Osteomyelitis. Other Infections e.g. septic abortion, puerperal sepsis, intra‐abdominal sepsis, septicaemia, peritonitis, post‐surgical infections. Synermox is indicated for prophylaxis against infection which may be associated with major surgical procedures such as those involving: Gastro‐intestinal tract Pelvic cavity 1 | Page Head and neck Cardiac Renal Joint replacement Biliary tract surgery Infections caused by amoxicillin susceptible organisms are amenable to Synermox treatment due to its amoxicillin content. Mixed infections caused by amoxicillin susceptible organisms in conjunction with Synermox‐susceptible beta‐lactamase‐producing organisms may therefore be treated by Synermox. -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment.