The Treatment of Melioidosis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Polymyxin B in Combination with Doripenem Against Heteroresistant Acinetobacter Baumannii: Pharmacodynamics of New Dosing Strategies
J Antimicrob Chemother 2016; 71: 3148–3156 doi:10.1093/jac/dkw293 Advance Access publication 3 August 2016 Polymyxin B in combination with doripenem against heteroresistant Acinetobacter baumannii: pharmacodynamics of new dosing strategies Gauri G. Rao1,2, Neang S. Ly1,2,Ju¨rgen B. Bulitta1,3, Rachel L. Soon1,2, Marie D. San Roman1,2, Patricia N. Holden1,2, Cornelia B. Landersdorfer4, Roger L. Nation4, Jian Li4, Alan Forrest1,5 and Brian T. Tsuji1,2* 1Laboratory for Antimicrobial Pharmacodynamics, School of Pharmacy and Pharmaceutical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, USA; 2The New York State Center of Excellence in Bioinformatics & Life Sciences, University at Buffalo, The State University of New York, Buffalo, NY, USA; 3Center for Pharmacometrics and Systems Pharmacology, College of Pharmacy, University of Florida, Orlando, FL, USA; 4Drug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, Australia; 5Eshelman School of Pharmacy, The University of North Carolina at Chapel Hill, NC, USA *Corresponding author. Tel: +1-716-881-7543; Fax: +1-716-849-6890; E-mail: [email protected] Received 13 October 2015; returned 3 January 2016; revised 14 June 2016; accepted 19 June 2016 Objectives: Polymyxin B is being increasingly utilized as a last resort against resistant Gram-negative bacteria. We examined the pharmacodynamics of novel dosing strategies for polymyxin B combinations to maximize efficacy and minimize the emergence of resistance and drug exposure against Acinetobacter baumannii. Methods: The pharmacodynamics of polymyxin B together with doripenem were evaluated in time–kill experi- ments over 48 h against 108 cfu/mL of two polymyxin-heteroresistant A. -

Ceftazidime for Injection) PHARMACY BULK PACKAGE – NOT for DIRECT INFUSION
PRESCRIBING INFORMATION FORTAZ® (ceftazidime for injection) PHARMACY BULK PACKAGE – NOT FOR DIRECT INFUSION To reduce the development of drug-resistant bacteria and maintain the effectiveness of FORTAZ and other antibacterial drugs, FORTAZ should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Ceftazidime is a semisynthetic, broad-spectrum, beta-lactam antibacterial drug for parenteral administration. It is the pentahydrate of pyridinium, 1-[[7-[[(2-amino-4 thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1 azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, hydroxide, inner salt, [6R-[6α,7β(Z)]]. It has the following structure: The molecular formula is C22H32N6O12S2, representing a molecular weight of 636.6. FORTAZ is a sterile, dry-powdered mixture of ceftazidime pentahydrate and sodium carbonate. The sodium carbonate at a concentration of 118 mg/g of ceftazidime activity has been admixed to facilitate dissolution. The total sodium content of the mixture is approximately 54 mg (2.3 mEq)/g of ceftazidime activity. The Pharmacy Bulk Package vial contains 709 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3mEq) per gram of ceftazidime. FORTAZ in sterile crystalline form is supplied in Pharmacy Bulk Packages equivalent to 6g of anhydrous ceftazidime. The Pharmacy Bulk Package bottle is a container of sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous use. THE PHARMACY BULK PACKAGE IS NOT FOR DIRECT INFUSION, FURTHER DILUTION IS REQUIRED BEFORE USE. -

Activity of Aztreonam in Combination with Ceftazidime-Avibactam Against
Diagnostic Microbiology and Infectious Disease 99 (2021) 115227 Contents lists available at ScienceDirect Diagnostic Microbiology and Infectious Disease journal homepage: www.elsevier.com/locate/diagmicrobio Activity of aztreonam in combination with ceftazidime–avibactam against serine- and metallo-β-lactamase–producing ☆ ☆☆ ★ Pseudomonas aeruginosa , , Michelle Lee a, Taylor Abbey a, Mark Biagi b, Eric Wenzler a,⁎ a College of Pharmacy, University of Illinois at Chicago, Chicago, IL, USA b College of Pharmacy, University of Illinois at Chicago, Rockford, IL, USA article info abstract Article history: Existing data support the combination of aztreonam and ceftazidime–avibactam against serine-β-lactamase Received 29 June 2020 (SBL)– and metallo-β-lactamase (MBL)–producing Enterobacterales, although there is a paucity of data against Received in revised form 15 September 2020 SBL- and MBL-producing Pseudomonas aeruginosa. In this study, 5 SBL- and MBL-producing P. aeruginosa (1 Accepted 19 September 2020 IMP, 4 VIM) were evaluated against aztreonam and ceftazidime–avibactam alone and in combination via broth Available online xxxx microdilution and time-kill analyses. All 5 isolates were nonsusceptible to aztreonam, aztreonam–avibactam, – – Keywords: and ceftazidime avibactam. Combining aztreonam with ceftazidime avibactam at subinhibitory concentrations Metallo-β-lactamase produced synergy and restored bactericidal activity in 4/5 (80%) isolates tested. These results suggest that the VIM combination of aztreonam and ceftazidime–avibactam may be a viable treatment option against SBL- and IMP MBL-producing P. aeruginosa. Aztreonam © 2020 Elsevier Inc. All rights reserved. Ceftazidime–avibactam Synergy 1. Introduction of SBLs and MBLs harbored by P. aeruginosa are fundamentally different than those in Enterobacterales (Alm et al., 2015; Kazmierczak et al., The rapid global dissemination of Ambler class B metallo-β- 2016; Periasamy et al., 2020; Poirel et al., 2000; Watanabe et al., lactamase (MBL) enzymes along with their increasing diversity across 1991). -

Pharmacodynamic Evaluation of Suppression of in Vitro Resistance In
www.nature.com/scientificreports OPEN Pharmacodynamic evaluation of suppression of in vitro resistance in Acinetobacter baumannii strains using polymyxin B‑based combination therapy Nayara Helisandra Fedrigo1, Danielle Rosani Shinohara1, Josmar Mazucheli2, Sheila Alexandra Belini Nishiyama1, Floristher Elaine Carrara‑Marroni3, Frederico Severino Martins4, Peijuan Zhu5, Mingming Yu6, Sherwin Kenneth B. Sy2 & Maria Cristina Bronharo Tognim1* The emergence of polymyxin resistance in Gram‑negative bacteria infections has motivated the use of combination therapy. This study determined the mutant selection window (MSW) of polymyxin B alone and in combination with meropenem and fosfomycin against A. baumannii strains belonging to clonal lineages I and III. To evaluate the inhibition of in vitro drug resistance, we investigate the MSW‑derived pharmacodynamic indices associated with resistance to polymyxin B administrated regimens as monotherapy and combination therapy, such as the percentage of each dosage interval that free plasma concentration was within the MSW (%TMSW) and the percentage of each dosage interval that free plasma concentration exceeded the mutant prevention concentration (%T>MPC). The MSW of polymyxin B varied between 1 and 16 µg/mL for polymyxin B‑susceptible strains. The triple combination of polymyxin B with meropenem and fosfomycin inhibited the polymyxin B‑resistant subpopulation in meropenem‑resistant isolates and polymyxin B plus meropenem as a double combination sufciently inhibited meropenem‑intermediate, and susceptible strains. T>MPC 90% was reached for polymyxin B in these combinations, while %TMSW was 0 against all strains. TMSW for meropenem and fosfomycin were also reduced. Efective antimicrobial combinations signifcantly reduced MSW. The MSW‑derived pharmacodynamic indices can be used for the selection of efective combination regimen to combat the polymyxin B‑resistant strain. -

Global Assessment of the Antimicrobial Activity of Polymyxin B Against 54 731 Clinical Isolates of Gram-Negative Bacilli
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ORIGINAL ARTICLE 10.1111/j.1469-0691.2005.01351.x Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004) A. C. Gales1, R. N. Jones2,3 and H. S. Sader1,2 1Division of Infectious Diseases, Universidade Federal de Sa˜o Paulo, Sa˜o Paulo, Brazil, 2JMI Laboratories, North Liberty, IA, USA, and 3Tufts University School of Medicine, Boston, MA, USA ABSTRACT In total, 54 731 Gram-negative bacilli isolated worldwide between 2001 and 2004 from diverse sites of infection were tested for susceptibility to polymyxin B by the broth reference microdilution method, with interpretation of results according to CLSI (formerly NCCLS) guidelines. Polymyxin B showed excellent potency and spectrum against 8705 Pseudomonas aeruginosa and 2621 Acinetobacter spp. isolates (MIC50, £ ⁄ ⁄ 1mg L and MIC90,2mg L for both pathogens). Polymyxin B resistance rates were slightly higher for carbapenem-resistant P. aeruginosa (2.7%) and Acinetobacter spp. (2.8%), or multidrug-resistant (MDR) P. aeruginosa (3.3%) and Acinetobacter spp. (3.2%), when compared with the entire group (1.3% for P. aeruginosa and 2.1% for Acinetobacter spp.). Among P. aeruginosa, polymyxin B resistance rates varied from 2.9% in the Asia-Pacific region to only 1.1% in Europe, Latin America and North America, while polymyxin B resistance rates ranged from 2.7% in Europe to 1.7% in North America and Latin America £ ⁄ % among Acinetobacter spp. -

1072 ICAAC Fritschen MW08 F
POSTER E-0115 Thomas R. Fritsche, MD, PhD In-vitro Activity of Ceftobiprole Tested Against a Recent Collection of JMI Laboratories ICAAC 2006 345 Beaver Kreek Centre, Suite A North American Pseudomonas aeruginosa North Liberty, IA 52317 Tel: (319) 665-3370 T.R. Fritsche, H.S. Sader, R.N. Jones • JMI Laboratories, North Liberty, Iowa, USA Fax: (319) 665-3371 e-mail: [email protected] Introduction Results Updated Abstract Table 1. In-vitro activity of ceftobiprole in comparison to selected antimicrobial agents tested against 221 isolates of P. aeruginosa Ceftobiprole (previously known as BAL9141), is an investigational broad- (North America) • P. aeruginosa isolates recovered from patients in North America generally Background: Ceftobiprole (BAL9141; BPR) is an investigational spectrum cephalosporin with potent activity against both Gram-negative and displayed less antibiotic resistance than did isolates from the all-isolate Antimicrobial agent MIC (µg/ml) MIC (µg/ml) Range (µg/ml) % Susceptible/resistanta cephalosporin with a broad spectrum of activity including methicillin- Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus 50 90 worldwide collection (examples: tobramycin, 3.6 and 20.8% resistance, resistant S. aureus. PK/PD characteristics of the parenteral prodrug (MRSA) (3, 7-9). The agent is stable to many β-lactamases and has a Ceftobiprole 2 >8 ≤0.06 – >8 - / - respectively; imipenem, 5.9 and 11.6%; Tables 1 and 2). formulation are consistent with usable potencies against streptococci, Ceftazidime 2 >16 ≤1 – >16 78.7 / 15.8 strong affinity for penicillin binding proteins (PBPs), including PBP2a, which • Ceftobiprole was as active as ceftazidime and cefepime (MIC , 2 µg/ml) E. -

A Phase I, 3-Part Placebo-Controlled Randomised Trial to Evaluate the Safety, Tolerability and Pharmacokinetics of Aztreonam-Av
EV0643 Contactinformation: A phase I, 3-part placebo-controlled randomised trial to evaluate the safety, SeamusO’Brien AstraZenecaResearch&Development Macclesfield,Cheshire,UK tolerability and pharmacokinetics of aztreonam-avibactam in healthy subjects [email protected] Timi Edeki1, Diansong Zhou2, Frans van den Berg3, Helen Broadhurst4, William C. Holmes5, Gary Peters3, Maria Sunzel6, Seamus O’Brien4 1AstraZeneca, Wilmington, DE, USA; 2AstraZeneca, Waltham, MA, USA; 3Hammersmith Medicines Research, London, UK; 4AstraZeneca, Macclesfield, UK; 5AstraZeneca, Gaithersburg, MD, USA; 6Contractor at AstraZeneca, Wilmington, DE, USA Background Table 1.Subjects’baselinecharacteristics Figure 1. Geometricmean(A)aztreonamand(B)avibactamconcentration-timeprofilesinPartAfollowingindividualandcombinedadministrationofaztreonam2000mgandavibactam600mgby1-hIVinfusion Part A Part B Part C • Theemergenceandglobaldisseminationofmetallo-β-lactamase(MBL)-producing (A) (B) Enterobacteriaceae,includingNDM-typeandVIM-type,whichareresistanttocarbapenems, Active Placebo Active Placebo Active Placebo 100 Aztreonam 2000 mg alone (n=7) 100 Avibactam 600 mg alone (n=8) representsanurgentthreattohumanhealthforwhichfewtreatmentoptionscurrentlyexist.1, 2 (n=8) (n=4) (n=40) (n=16) (n=18) (n=6) Aztreonam-avibactam 2000-600 mg (n=7) Aztreonam-avibactam 2000-600 mg (n=7) • Aztreonam,amonobactamβ-lactam,isstabletoAmblerclassBMBLsincludingNDM-typeand Mean(SD)age,years 31(7) 34(7) 30(7) 27(5) 49(19) 51(19) VIM-type.However,bacteriathatexpressMBLsfrequentlyco-expressotherclassesofβ-lactamase -

General Items
Essential Medicines List (EML) 2019 Application for the inclusion of imipenem/cilastatin, meropenem and amoxicillin/clavulanic acid in the WHO Model List of Essential Medicines, as reserve second-line drugs for the treatment of multidrug-resistant tuberculosis (complementary lists of anti-tuberculosis drugs for use in adults and children) General items 1. Summary statement of the proposal for inclusion, change or deletion This application concerns the updating of the forthcoming WHO Model List of Essential Medicines (EML) and WHO Model List of Essential Medicines for Children (EMLc) to include the following medicines: 1) Imipenem/cilastatin (Imp-Cln) to the main list but NOT the children’s list (it is already mentioned on both lists as an option in section 6.2.1 Beta Lactam medicines) 2) Meropenem (Mpm) to both the main and the children’s lists (it is already on the list as treatment for meningitis in section 6.2.1 Beta Lactam medicines) 3) Clavulanic acid to both the main and the children’s lists (it is already listed as amoxicillin/clavulanic acid (Amx-Clv), the only commercially available preparation of clavulanic acid, in section 6.2.1 Beta Lactam medicines) This application makes reference to amendments recommended in particular to section 6.2.4 Antituberculosis medicines in the latest editions of both the main EML (20th list) and the EMLc (6th list) released in 2017 (1),(2). On the basis of the most recent Guideline Development Group advising WHO on the revision of its guidelines for the treatment of multidrug- or rifampicin-resistant (MDR/RR-TB)(3), the applicant considers that the three agents concerned be viewed as essential medicines for these forms of TB in countries. -

Severe Sepsis and Septic Shock Antibiotic Guide
Stanford Health Issue Date: 05/2017 Stanford Antimicrobial Safety and Sustainability Program Severe Sepsis and Septic Shock Antibiotic Guide Table 1: Antibiotic selection options for healthcare associated and/or immunocompromised patients • Healthcare associated: intravenous therapy, wound care, or intravenous chemotherapy within the prior 30 days, residence in a nursing home or other long-term care facility, hospitalization in an acute care hospital for two or more days within the prior 90 days, attendance at a hospital or hemodialysis clinic within the prior 30 days • Immunocompromised: Receiving chemotherapy, known systemic cancer not in remission, ANC <500, severe cell-mediated immune deficiency Table 2: Antibiotic selection options for community acquired, immunocompetent patients Table 3: Antibiotic selection options for patients with simple sepsis, community acquired, immunocompetent patients requiring hospitalization. Risk Factors for Select Organisms P. aeruginosa MRSA Invasive Candidiasis VRE (and other resistant GNR) Community acquired: • Known colonization with MDROs • Central venous catheter • Liver transplant • Prior IV antibiotics within 90 day • Recent MRSA infection • Broad-spectrum antibiotics • Known colonization • Known colonization with MDROs • Known MRSA colonization • + 1 of the following risk factors: • Prolonged broad antibacterial • Skin & Skin Structure and/or IV access site: ♦ Parenteral nutrition therapy Hospital acquired: ♦ Purulence ♦ Dialysis • Prolonged profound • Prior IV antibiotics within 90 days ♦ Abscess -

Possible Clinical Indications of Ceftobiprole
©The Author 2019. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/). Ceftobiprole review José Barberán Possible clinical indications of ceftobiprole Servicio de Medicina Interna - Enfermedades infecciosas, Hospital Universitario HM Montepríncipe, Universidad San Pablo CEU. Madrid, Spain ABSTRACT demonstrated in vitro activity on the majority of Gram-positive cocci and aerobic Gram-negative bacilli of clinical relevance. Ceftobiprole is a fifth-generation cephalosporin approved On the former, it has heightened bactericidal action and in- for the treatment of adult community-acquired pneumonia cludes: 1) Staphylococcus spp., both methicillin- and vancomy- and non-ventilator associated hospital-acquired pneumonia. cin-resistant Staphylococcus aureus and coagulase-negative However, its microbiological and pharmacokinetic profile is staphylococci, 2) Streptococcus spp., including Streptococ- very attractive as armamentarium for empirical monotherapy cus pneumoniae resistant to penicillins and third-generation treatment in other infections too. Among these, the following cephalosporins, and 3) Enterococcus faecalis, as it is the first scenarios could be considered complicated skin and soft tissue and only cephalosporin here with demonstrated activity. With infections, moderate-severe diabetic foot infections without regard to Gram-negative bacilli, its spectrum includes -
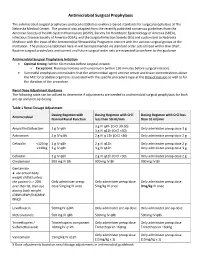
Antimicrobial Surgical Prophylaxis
Antimicrobial Surgical Prophylaxis The antimicrobial surgical prophylaxis protocol establishes evidence-based standards for surgical prophylaxis at The Nebraska Medical Center. The protocol was adapted from the recently published consensus guidelines from the American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America (SHEA), Infectious Disease Society of America (IDSA), and the Surgical Infection Society (SIS) and customized to Nebraska Medicine with the input of the Antimicrobial Stewardship Program in concert with the various surgical groups at the institution. The protocol established here-in will be implemented via standard order sets utilized within One Chart. Routine surgical prophylaxis and current and future surgical order sets are expected to conform to this guidance. Antimicrobial Surgical Prophylaxis Initiation Optimal timing: Within 60 minutes before surgical incision o Exceptions: Fluoroquinolones and vancomycin (within 120 minutes before surgical incision) Successful prophylaxis necessitates that the antimicrobial agent achieve serum and tissue concentrations above the MIC for probable organisms associated with the specific procedure type at the time of incision as well as for the duration of the procedure. Renal Dose Adjustment Guidance The following table can be utilized to determine if adjustments are needed to antimicrobial surgical prophylaxis for both pre-op and post-op dosing. Table 1 Renal Dosage Adjustment Dosing Regimen with Dosing Regimen with CrCl Dosing Regimen with -

Penicillin Allergy Guidance Document
Penicillin Allergy Guidance Document Key Points Background Careful evaluation of antibiotic allergy and prior tolerance history is essential to providing optimal treatment The true incidence of penicillin hypersensitivity amongst patients in the United States is less than 1% Alterations in antibiotic prescribing due to reported penicillin allergy has been shown to result in higher costs, increased risk of antibiotic resistance, and worse patient outcomes Cross-reactivity between truly penicillin allergic patients and later generation cephalosporins and/or carbapenems is rare Evaluation of Penicillin Allergy Obtain a detailed history of allergic reaction Classify the type and severity of the reaction paying particular attention to any IgE-mediated reactions (e.g., anaphylaxis, hives, angioedema, etc.) (Table 1) Evaluate prior tolerance of beta-lactam antibiotics utilizing patient interview or the electronic medical record Recommendations for Challenging Penicillin Allergic Patients See Figure 1 Follow-Up Document tolerance or intolerance in the patient’s allergy history Consider referring to allergy clinic for skin testing Created July 2017 by Macey Wolfe, PharmD; John Schoen, PharmD, BCPS; Scott Bergman, PharmD, BCPS; Sara May, MD; and Trevor Van Schooneveld, MD, FACP Disclaimer: This resource is intended for non-commercial educational and quality improvement purposes. Outside entities may utilize for these purposes, but must acknowledge the source. The guidance is intended to assist practitioners in managing a clinical situation but is not mandatory. The interprofessional group of authors have made considerable efforts to ensure the information upon which they are based is accurate and up to date. Any treatments have some inherent risk. Recommendations are meant to improve quality of patient care yet should not replace clinical judgment.