Assessing the Self-Purification Capacity of Surface Waters in Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
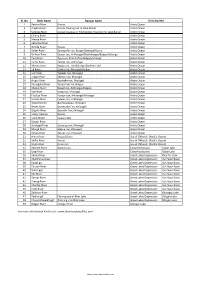
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -

The Influence of Climatic Change and Human Activity on Erosion Processes in Sub-Arid Watersheds in Southern East Siberia
HYDROLOGICAL PROCESSES Hydrol. Process. 17, 3181–3193 (2003) Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/hyp.1382 The influence of climatic change and human activity on erosion processes in sub-arid watersheds in southern East Siberia Leonid M. Korytny,* Olga I. Bazhenova, Galina N. Martianova and Elena A. Ilyicheva Institute of Geography, Siberian Branch of the Russian Academy of Sciences, 1 Ulanbatorskaya St., Irkutsk, 664033, Russia Abstract: A LUCIFS model variant is presented that represents the influence of climate and land use change on fluvial systems. The study considers trends of climatic characteristics (air temperature, annual precipitation totals, rainfall erosion index, aridity and continentality coefficients) for the steppe and partially wooded steppe watersheds of the south of East Siberia (the Yenisey River macro-watershed). It also describes the influence of these characteristics on erosion processes, one indicator of which is the suspended sediment yield. Changes in the river network structure (the order of rivers, lengths, etc.) as a result of agricultural activity during the 20th century are investigated by means of analysis of maps of different dates for one of the watersheds, that of the Selenga River, the biggest tributary of Lake Baikal. The study reveals an increase of erosion process intensity in the first two-thirds of the century in the Selenga River watershed and a reduction of this intensity in the last third of the century, both in the Selenga River watershed and in most of the other watersheds of the study area. Copyright 2003 John Wiley & Sons, Ltd. KEY WORDS LUCIFS; Yenisey watershed; fluvial system; climate change; land use change; river network structure; erosion processes. -

Trans-Baykal (Rusya) Bölgesi'nin Coğrafyasi
International Journal of Geography and Geography Education (IGGE) To Cite This Article: Can, R. R. (2021). Geography of the Trans-Baykal (Russia) region. International Journal of Geography and Geography Education (IGGE), 43, 365-385. Submitted: October 07, 2020 Revised: November 01, 2020 Accepted: November 16, 2020 GEOGRAPHY OF THE TRANS-BAYKAL (RUSSIA) REGION Trans-Baykal (Rusya) Bölgesi’nin Coğrafyası Reyhan Rafet CAN1 Öz Zabaykalskiy Kray (Bölge) olarak isimlendirilen saha adını Rus kâşiflerin ilk kez 1640’ta karşılaştıkları Daur halkından alır. Rusçada Zabaykalye, Balkal Gölü’nün doğusu anlamına gelir. Trans-Baykal Bölgesi, Sibirya'nın en güneydoğusunda, doğu Trans-Baykal'ın neredeyse tüm bölgesini işgal eder. Bölge şiddetli iklim koşulları; birçok mineral ve hammadde kaynağı; ormanların ve tarım arazilerinin varlığı ile karakterize edilir. Rusya Federasyonu'nun Uzakdoğu Federal Bölgesi’nin bir parçası olan on bir kurucu kuruluşu arasında bölge, alan açısından altıncı, nüfus açısından dördüncü, bölgesel ürün üretimi açısından (GRP) altıncı sıradadır. Bölge topraklarından geçen Trans-Sibirya Demiryolu yalnızca Uzak Doğu ile Rusya'nın batı bölgeleri arasında bir ulaşım bağlantısı değil, aynı zamanda Avrasya geçişini sağlayan küresel altyapının da bir parçasıdır. Bölgenin üretim yapısında sanayi, tarım ve ulaşım yüksek bir paya sahiptir. Bu çalışmada Trans-Baykal Bölgesi’nin fiziki, beşeri ve ekonomik coğrafya özellikleri ele alınmıştır. Trans-Baykal Bölgesinin coğrafi özelliklerinin yanı sıra, ekonomik ve kültürel yapısını incelenmiştir. Bu kapsamda konu ile ilgili kurumsal raporlardan ve alan araştırmalarından yararlanılmıştır. Bu çalışma sonucunda 350 yıldan beri Rus gelenek, kültür ve yaşam tarzının devam ettiği, farklı etnik grupların toplumsal birliği sağladığı, yer altı kaynaklarının bölge ekonomisi için yüzyıllardır olduğu gibi günümüzde de önem arz ettiği, coğrafyasının halkın yaşam şeklini belirdiği sonucuna varılmıştır. -

Subject of the Russian Federation)
How to use the Atlas The Atlas has two map sections The Main Section shows the location of Russia’s intact forest landscapes. The Thematic Section shows their tree species composition in two different ways. The legend is placed at the beginning of each set of maps. If you are looking for an area near a town or village Go to the Index on page 153 and find the alphabetical list of settlements by English name. The Cyrillic name is also given along with the map page number and coordinates (latitude and longitude) where it can be found. Capitals of regions and districts (raiony) are listed along with many other settlements, but only in the vicinity of intact forest landscapes. The reader should not expect to see a city like Moscow listed. Villages that are insufficiently known or very small are not listed and appear on the map only as nameless dots. If you are looking for an administrative region Go to the Index on page 185 and find the list of administrative regions. The numbers refer to the map on the inside back cover. Having found the region on this map, the reader will know which index map to use to search further. If you are looking for the big picture Go to the overview map on page 35. This map shows all of Russia’s Intact Forest Landscapes, along with the borders and Roman numerals of the five index maps. If you are looking for a certain part of Russia Find the appropriate index map. These show the borders of the detailed maps for different parts of the country. -

Download Download
© Entomologica Fennica. 15 June 2003 Contribution to the scythridid fauna of southern Buryatia, with description of seven new species (Lepidoptera: Scythrididae) Kari Nupponen Nupponen, K. 2003: Contribution to the scythridid fauna of southern Buryatia, with description of seven new species (Lepidoptera: Scythrididae). — Entomol. Fennica 14: 25–45. A list of 26 species embracing 673 specimens of the family Scythrididae collected during 13.–29.VI.2002 from southern Buryatia is presented. Seven new species are described: Scythris erinacella sp. n., S. gorbunovi sp. n., S. hamardabanica sp. n., S. malozemovi sp. n., S. ninae sp. n., S. potatorella sp. n. and S. sinevi sp. n. Two unknown species are mentioned but not described because only females are available. In addition, S. penicillata Chrétien, 1900 is reported as new for Russia, S. emichi (Anker, 1870) as new for the Asiatic part of Russia and seven further species as new for the Baikal region. The known distribution range of each species is given as well as further notes on some poorly known taxa. Kari Nupponen, Miniatontie 1 B 9, FIN-02360 Espoo, Finland Received 30 October 2002, accepted 21 January 2003 1. Introduction & Liòka 1996, Sinev 2001) concern occasionally collected single specimens, and no systematic in- The Republic of Buryatia is located on the southern vestigations of scythridids have been made in the and southeastern shores of Lake Baikal. Character- region. The present article is based on material istic features of the southern part of that region are collected by the author during 13.–29.VI.2002 in mountain ranges: East Sayan Mountains in the west southern Buryatia. -

Sustainable Water Management in the Selenga-Baikal Basin
Sustainable Water Management in the Selenga-Baikal Basin Integrated Environmental Assessment for a Transboundary Watershed with Multiple Stressors Contact Integrated Water Resources Management – Model Region Mongolia – If you have any questions or suggestions, please do not hesitate to contact us: Dr. Daniel Karthe | [email protected] Philipp Theuring | [email protected] Helmholtz Centre for Environmental Research, Magdeburg, Germany Acad. Prof. Dr. Nikolay Kasimov | [email protected] Dr. Sergey Chalov | [email protected] Lomonosov Moscow State University, Russia For further information and contacts, please refer to www.iwrm-momo.de Project Profi le Sustainable Water Management in the Selenga-Baikal Basin Integrated Environmental Assessment for a Transboundary Watershed with Multiple Stressors August 2014 Publisher: Helmholtz Centre for Environmental Research (UFZ) Moscow Lomonosov State University Editors: Dr. Daniel Karthe | Dr. Sergey Chalov Layout: perner&schmidt werbung und design gmbh Sponsors: International Bureau of the German Federal Ministry of Education and Research (BMBF) Russian Geographical Society Links: www.ufz.de www.eng.geogr.msu.ru SELENGA-BAIKAL PROJECT Lake Baikal Page 2 SELENGA-BAIKAL PROJECT Table of Contents/Partners Table of Contents Page 1 Introduction . 4 2 Hydrochemical Monitoring in the Selenga-Baikal Basin . 5 3 Planning IWRM in the Kharaa River Model Region . 6 4 A Monitoring Concept for the Selenga-Baikal Basin . 7 5 Selected Publications . 8 Contact . 9 Partners Helmholtz Centre for Environmental Research Lomonosov Moscow State University Associated partners are the Mongolian Academy of Sciences (Institutes of Geograpy and Geoecology), the Russian Academy of Sciences (Institute of Water Problems, the Joint Russian-Mongolian Complex Biological Expedition) and the Center for Environmental Systems Research of Kassel University, Germany. -

Supplement of Geosci
Supplement of Geosci. Model Dev., 11, 1343–1375, 2018 https://doi.org/10.5194/gmd-11-1343-2018-supplement © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement of LPJmL4 – a dynamic global vegetation model with managed land – Part 1: Model description Sibyll Schaphoff et al. Correspondence to: Sibyll Schaphoff ([email protected]) The copyright of individual parts of the supplement might differ from the CC BY 4.0 License. S1 Supplementary informations to the evaluation of the LPJmL4 model The here provided supplementary informations give more details to the evaluations given in Schaphoff et al.(under Revision). All sources and data used are described in detail there. Here we present ad- ditional figures for evaluating the LPJmL4 model on a plot scale for water and carbon fluxes Fig. S1 5 - S16. Here we use the standard input as described by Schaphoff et al.(under Revision, Section 2.1). Furthermore, we evaluate the model performance on eddy flux tower sites by using site specific me- teorological input data provided by http://fluxnet.fluxdata.org/data/la-thuile-dataset/(ORNL DAAC, 2011). Here the long time spin up of 5000 years was made with the input data described in Schaphoff et al.(under Revision), but an additional spin up of 30 years was conducted with the site specific 10 input data followed by the transient run given by the observation period. Comparisons are shown for some illustrative stations for net ecosystem exchange (NEE) in Fig. S17 and for evapotranspira- tion Fig. S18. -

Chemical Water Quality in Selenge River Basin in Mongolia: Spatial-Temporal Patterns and Land Use Influence
Chemical water quality in Selenge River Basin in Mongolia: spatial-temporal patterns and land use influence DISSERTATION for the award of the degree “Doctor rerum naturalium” (Dr.rer.nat.) of the Georg-August-Universität Göttingen within the doctoral program HIGRADE of the “Helmholtz- Centre for Environmental Research-UFZ” Submitted by Gunsmaa Batbayar (MSc) From Mongolia Göttingen, June 2018 Thesis Committee Prof. Dr. Martin Kappas Department of Cartography, GIS and Remote Sensing, institute of Geography, University of Göttingen Prof. Dr. Daniel Karthe Environmental Engineering Section, German-Mongolian Institute for Resources and Technology (GMIT) Members of the Examination Board Reviewer: Prof. Dr. Martin Kappas, Department of Cartography, GIS and Remote Sensing, Institute of Geography, University of Göttingen Second Reviewer: Prof. Dr. Daniel Karthe Environmental Engineering Section, German-Mongolian Institute for Resources and Technology (GMIT) Further members of the Examination Board PD Dr. Dr. h.c. Martin Pfeiffer, Bayreuth Center of Ecology and Environmental Research BayCEER, University of Bayreuth Prof. Dr. Daniela Sauer, Abteilung Physische Geographie, Georg-August Universität Göttingen Prof. Dr. Hans Ruppert, Georg-August-Universität, Geowissenschaftliches Zentrum, Dept. Sedimentologie/Umweltgeologie Dr. Michael Schlund, Department of Cartography, GIS and Remote Sensing, Institute of Geography, University of Göttingen Date of the oral examination: 09.07.2018 Acknowledgments First of all, I would like to express my sincere thanks to my supervisors PD Dr. Dr. h.c. Martin Pfeiffer, Prof. Dr. Daniel Karthe, Prof. Dr. Martin Kappas, and Prof. Dr. Dietrich Borchardt for offering me this wonderful opportunity to join the UFZ community in Magdeburg and Georg-August University Göttingen in Germany. Thank you for your continuous supervision, support and advice which helped me to successfully complete this research and I would like to say that without your help it was not possible to finalize. -

Water Resources Assessment of the Selenga-Baikal River System
GEOÖKO VOLUME/BAND XXXIV, 77 – 102 GÖTTINGEN 2013 WATER RESOURCES ASSESSMENT OF THE SELENGA-BAIKAL RIVER SYSTEM SERGEY CHALOV, NIKOLAY KASIMOV, MIKHAIL LYCHAGIN, EKATERINA BELOZEROVA, GALINA SHINKAREVA, PHILIPP THEURING, ANNA ROMANCHENKO, NIKOLAY ALEXEEVSKY, ENDON GARMAEV SUMMARY Paper provides basin-scale integrated assessment of water resources of the largest tributary of Lake Baikal (the Selenga River) extending from northern Mongolia into southern Siberia in Russia with special emphasis on total flow of water through a basin, its quality, structures, laws and economic factors that control its use for the present and future. The water quality and flow data were obtained from historical measurement campaigns, long-term national monitoring, and a novel field campaign done in 2011-2012. The results provide evidence on a very low water availability in upper parts of the basin and insufficient water quality exhibiting high concentrations of dissolved and suspended heavy metals in downstream parts of the Selenga River where elevated concentrations of dissolved and suspended forms of Mn, Fe, Cu, Mo are observed with local 10-20-fold increases of maximum permissible concentrations MPC below large industrial and mining centers. The main pressure on water resources is reported for some Mongolian (Tuul below Ulanbaatar and Zaamar goldfield and the Khangal River below copper-molybdenum mine-mill complex Erdenet) and Russian rivers (Modonkul-Dzhida River system below Zakamensk wolfram-molybdenum mining and processing factory). We concluded that future water resources will depend on general socio-economic trends in both Mongolia and Russia with special focus on development of mining concessions which overlap with current or planned hydropower projects and popu- lation changes and thus water consumption in the region. -

The Nature Conservation of Baikal Region: Special Natural Protected Areas System in Three Environmental Models
9 The Nature Conservation of Baikal Region: Special Natural Protected Areas System in Three Environmental Models Tatyana P. Kalikhman Institute of Geography of Siberian Branch of Russian Academy of Sciences, Irkutsk Russia 1. Introduction Lake Baikal is one of the most unique places on Earth. This is the oldest and deepest lake in the world. It contains 20 % of the world’s fresh water and is home to more than 2,000 endemic species of plants and animals. Therefore, current conservation of the waters of Lake Baikal and the surrounding region, is affecting the state of its ecosystem. In 1996, the Baikal area and its immediate surroundings were included in the list of World Natural Heritage Sites by UNESCO. In 1999, a special federal law "On protection of Lake Baikal» was passed. This is Russia's only law regarding the management of the lake and the Baikal region. The main way to preserve, maintain and restore the biotic and landscape diversity is through territorial nature conservation. The territorial nature conservation activities are all of the efforts to protect areas of different categories, status and regimes of protection. In accordance with federal law "On specially protected natural areas», the main categories of protected areas are zapovednik (nature reserves or strictly protected areas), natural park (regionally declared), national park (federally declared), and zakaznik (refuges established at the federal and regional levels). There are also other, less significant (in terms of conservation) categories of protected areas. The two most widely used approaches to conservation of protected areas are: the basin approach and administrative approach. -

Degruyter Opar Opar-2020-0135 155..176 ++
Open Archaeology 2021; 7: 155–176 Research Article Irina Alexandrovna Ponomareva* Exploring the Cave Rock Art of Siberian Trans-Baikal: Fertility, Shamanism, and Gender https://doi.org/10.1515/opar-2020-0135 received October 1, 2020; accepted March 19, 2021 Abstract: The paper explores the phenomenon of rock art found in and around rock art cavities in Trans-Baikal region of South-East Siberia. Although many researchers noticed that caves have had a special value in cultures around the globe, no research has been carried out specifically into the cave rock art of Trans-Baikal which was not distinguished from other rock art found in open localities and shelters. This study was conducted based on field data collected by the author in 2017. In order to answer the question whether the cave sites had a specific role in the cultures of Bronze Age Trans-Baikal, the sets of motifs of the cave sites were compared to those of the closest open sites. Drawing on the stylistic difference revealed by the analysis and landscapecontext,itissuggestedthatthecaverockartsitescouldbeplaceswhereritualsofmorerestricted nature took place. Ethnographic records may imply that these ceremonies were aimed at the fertility increasing being performed by shamans or shamannesses or without their assistance. It is also possible that the ceremonies could be gender-exclusive, conducted only for women, although this interpretation needs further research. Keywords: Siberia, rock art, caves, Bronze Age 1 Introduction 1.1 Significance of the Cave Setting, the Definition of Cave, and the Novelty of the Research Around the world, caves were noticed to exhibit a special value in religious life of different societies and were places where various rituals were performed (see David, 2017; Moyes, 2012b). -

08 Endon Garmaev
RUSSIAN ACADEMY OF SCIENCES SIBERIAN BRANCH BAIKAL INSTITUTE OF NATURE MANAGEMENT Garmaev Endon Zh. ScD (Geography), Professor of the RAS, Director of the BINM SB RAS Istomino, Buryatia, Russia, 2017 1 Lake Baikal together with the adjacent territories in 1996 was included in the UNESCO World Heritage List. In connection with this, the problem of the protection and conservation of lake water resources is acquired exclusively acute. The Baikal Lake basin is located almost in the center of the vast Asian continent 45% of which lies within the Russian Federation, the rest is on the territory of Mongolia. About 73% of river waters are formed on the territory of Russia, 27% – Mongolia. The Selenga river basin: 67 % - on Mongolian territory, 33 % - Russian. Baikal Nature Territory and Lake Baikal Basin 2 Density of rivers network of Lake Baikal basin 3 Problems of the lake Baikal Ecological problems: •Absence of an effective system of waste management (landfills, utilization, processing of solid waste, discharges of liquid waste and oil pollution by water transport); •Poor quality of wastewater purification (obsolete purification facilities or their complete absence); •High level of air pollution (wind rose, boiler houses, transport, dirt roads); •Forest and peat fires (climate, human factor, fire prevention measures, fire monitoring and forecasting center); •Deforestation (illegal woodcutting in the Lake Baikal basin and prohibition of sanitary felling in forests located in the central ecological zone); •The level regime of the lake Baikal