Developments in Environmental Biology of Fishes 17 Series Editor EUGENE K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Diversity of Fish (Pisces) in Freshwater
Hydrobiologia (2008) 595:545–567 DOI 10.1007/s10750-007-9034-0 FRESHWATER ANIMAL DIVERSITY ASSESSMENT Global diversity of fish (Pisces) in freshwater C. Le´veˆque Æ T. Oberdorff Æ D. Paugy Æ M. L. J. Stiassny Æ P. A. Tedesco Ó Springer Science+Business Media B.V. 2007 Abstract The precise number of extant fish spe- species live in lakes and rivers that cover only 1% cies remains to be determined. About 28,900 species of the earth’s surface, while the remaining 16,000 were listed in FishBase in 2005, but some experts species live in salt water covering a full 70%. While feel that the final total may be considerably higher. freshwater species belong to some 170 families (or Freshwater fishes comprise until now almost 13,000 207 if peripheral species are also considered), the species (and 2,513 genera) (including only fresh- bulk of species occur in a relatively few groups: water and strictly peripheral species), or about the Characiformes, Cypriniformes, Siluriformes, 15,000 if all species occurring from fresh to and Gymnotiformes, the Perciformes (noteably the brackishwaters are included. Noteworthy is the fact family Cichlidae), and the Cyprinodontiformes. that the estimated 13,000 strictly freshwater fish Biogeographically the distribution of strictly fresh- water species and genera are, respectively 4,035 species (705 genera) in the Neotropical region, 2,938 (390 genera) in the Afrotropical, 2,345 (440 Guest editors: E. V. Balian, C. Le´veˆque, H. Segers & K. Martens genera) in the Oriental, 1,844 (380 genera) in the Freshwater Animal Diversity Assessment Palaearctic, 1,411 (298 genera) in the Nearctic, and 261 (94 genera) in the Australian. -

JVP 26(3) September 2006—ABSTRACTS
Neoceti Symposium, Saturday 8:45 acid-prepared osteolepiforms Medoevia and Gogonasus has offered strong support for BODY SIZE AND CRYPTIC TROPHIC SEPARATION OF GENERALIZED Jarvik’s interpretation, but Eusthenopteron itself has not been reexamined in detail. PIERCE-FEEDING CETACEANS: THE ROLE OF FEEDING DIVERSITY DUR- Uncertainty has persisted about the relationship between the large endoskeletal “fenestra ING THE RISE OF THE NEOCETI endochoanalis” and the apparently much smaller choana, and about the occlusion of upper ADAM, Peter, Univ. of California, Los Angeles, Los Angeles, CA; JETT, Kristin, Univ. of and lower jaw fangs relative to the choana. California, Davis, Davis, CA; OLSON, Joshua, Univ. of California, Los Angeles, Los A CT scan investigation of a large skull of Eusthenopteron, carried out in collaboration Angeles, CA with University of Texas and Parc de Miguasha, offers an opportunity to image and digital- Marine mammals with homodont dentition and relatively little specialization of the feeding ly “dissect” a complete three-dimensional snout region. We find that a choana is indeed apparatus are often categorized as generalist eaters of squid and fish. However, analyses of present, somewhat narrower but otherwise similar to that described by Jarvik. It does not many modern ecosystems reveal the importance of body size in determining trophic parti- receive the anterior coronoid fang, which bites mesial to the edge of the dermopalatine and tioning and diversity among predators. We established relationships between body sizes of is received by a pit in that bone. The fenestra endochoanalis is partly floored by the vomer extant cetaceans and their prey in order to infer prey size and potential trophic separation of and the dermopalatine, restricting the choana to the lateral part of the fenestra. -
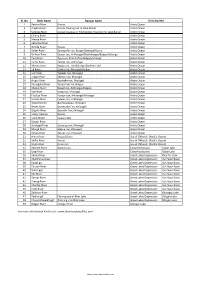
List of Rivers of Mongolia
Sl. No River Name Russian Name Draining Into 1 Yenisei River Russia Arctic Ocean 2 Angara River Russia, flowing out of Lake Baikal Arctic Ocean 3 Selenge River Сэлэнгэ мөрөн in Sükhbaatar, flowing into Lake Baikal Arctic Ocean 4 Chikoy River Arctic Ocean 5 Menza River Arctic Ocean 6 Katantsa River Arctic Ocean 7 Dzhida River Russia Arctic Ocean 8 Zelter River Зэлтэрийн гол, Bulgan/Selenge/Russia Arctic Ocean 9 Orkhon River Орхон гол, Arkhangai/Övörkhangai/Bulgan/Selenge Arctic Ocean 10 Tuul River Туул гол, Khentii/Töv/Bulgan/Selenge Arctic Ocean 11 Tamir River Тамир гол, Arkhangai Arctic Ocean 12 Kharaa River Хараа гол, Töv/Selenge/Darkhan-Uul Arctic Ocean 13 Eg River Эгийн гол, Khövsgöl/Bulgan Arctic Ocean 14 Üür River Үүрийн гол, Khövsgöl Arctic Ocean 15 Uilgan River Уйлган гол, Khövsgöl Arctic Ocean 16 Arigiin River Аригийн гол, Khövsgöl Arctic Ocean 17 Tarvagatai River Тарвагтай гол, Bulgan Arctic Ocean 18 Khanui River Хануй гол, Arkhangai/Bulgan Arctic Ocean 19 Ider River Идэр гол, Khövsgöl Arctic Ocean 20 Chuluut River Чулуут гол, Arkhangai/Khövsgöl Arctic Ocean 21 Suman River Суман гол, Arkhangai Arctic Ocean 22 Delgermörön Дэлгэрмөрөн, Khövsgöl Arctic Ocean 23 Beltes River Бэлтэсийн Гол, Khövsgöl Arctic Ocean 24 Bügsiin River Бүгсийн Гол, Khövsgöl Arctic Ocean 25 Lesser Yenisei Russia Arctic Ocean 26 Kyzyl-Khem Кызыл-Хем Arctic Ocean 27 Büsein River Arctic Ocean 28 Shishged River Шишгэд гол, Khövsgöl Arctic Ocean 29 Sharga River Шарга гол, Khövsgöl Arctic Ocean 30 Tengis River Тэнгис гол, Khövsgöl Arctic Ocean 31 Amur River Russia/China -
Íopicos E Noticias Onde Estamos? Para Onde Vamos?
Í'|BÍ"' da Director-EDMUNDO BITTENCOURT .umi ii ini» imim egBwwg DE 1904 Redaccâo—Rua Moreira César ti. 117 Anno IV—N. 1.162 RIO DE JANEIRO-QUINTA-FEIRA, 18 DE AGOSTO .P_j_lCE_»C____M___5 se á força cre_r contra o diversos especta- ç5es que quer ASSIGNATURAS serviuior do órgão nüo cábeii responsabilidade Algumas balas feriram assucar da Companhia Assucareira. Todos fm-io, vi-t,. li.vid-, tempo o do nào ter para dores. deste assttçár tecem- AGGRESSAO Anno 301000 concerto do instrumento. quantos tem usado completo Entre o numero de que estamos? grande pessoas Onde lhe cticomios. e acreditamos que 8eís mezes 18*000 mais ou menos justos .Io da Republica esti- soffreram contusões, gra- fará brilhante carreira, devido Numoro atrazado 100 réis No gabinete presidente Em Port Arthur A Allemanha e os ulti- elle princi- verãui o deputado Mello Mattos, uenera) Her- ves, conta-se o vice-presidente do Sena- mos acontecimentos—O cruzador «Dia- palmente á propaganda dos freguezes sa- nte . du .-_.,.s»vn, dr. Noemio da Silveira e dr. dia do, marquez de Pidal, que ficou ferido no SR. YARELA na» Pormenores do combate do Para onde vamos? tisfeitissimos. AO Cesário Pereira. 10-A Argentina e a guerra-A trage- rosto. A Companhia Assucareira ha de lutar O dr. Utinldino do Aniiirai, dir. ctor do Banco dia do «Rurik»-Notas avulsas. O marquez de Pidal recebeu, no rosto, O illustre deputado Alfredo Varela vae e lutar muito, entretanto nao duvidamos dn Republica, esteve em conferência com o uma bala até agora, uão foi possível ministro da fazenda sobre a da Soroca- que, um anno repudiou a do seu triumpho, porque nlo lia exemplo A tiros dc revólver—Na rua do questão S. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

The Influence of Climatic Change and Human Activity on Erosion Processes in Sub-Arid Watersheds in Southern East Siberia
HYDROLOGICAL PROCESSES Hydrol. Process. 17, 3181–3193 (2003) Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/hyp.1382 The influence of climatic change and human activity on erosion processes in sub-arid watersheds in southern East Siberia Leonid M. Korytny,* Olga I. Bazhenova, Galina N. Martianova and Elena A. Ilyicheva Institute of Geography, Siberian Branch of the Russian Academy of Sciences, 1 Ulanbatorskaya St., Irkutsk, 664033, Russia Abstract: A LUCIFS model variant is presented that represents the influence of climate and land use change on fluvial systems. The study considers trends of climatic characteristics (air temperature, annual precipitation totals, rainfall erosion index, aridity and continentality coefficients) for the steppe and partially wooded steppe watersheds of the south of East Siberia (the Yenisey River macro-watershed). It also describes the influence of these characteristics on erosion processes, one indicator of which is the suspended sediment yield. Changes in the river network structure (the order of rivers, lengths, etc.) as a result of agricultural activity during the 20th century are investigated by means of analysis of maps of different dates for one of the watersheds, that of the Selenga River, the biggest tributary of Lake Baikal. The study reveals an increase of erosion process intensity in the first two-thirds of the century in the Selenga River watershed and a reduction of this intensity in the last third of the century, both in the Selenga River watershed and in most of the other watersheds of the study area. Copyright 2003 John Wiley & Sons, Ltd. KEY WORDS LUCIFS; Yenisey watershed; fluvial system; climate change; land use change; river network structure; erosion processes. -

First Record of the North American Paddlefish (Polyodon Spathula Walbaum, 1792) in the Serbian Part of the Danube River
Arch. Biol. Sci., Belgrade, 58 (3), 27P-28P, 2006. FIRST RECORD OF THE NORTH AMERICAN PADDLEFISH (POLYODON SPATHULA WALBAUM, 1792) IN THE SERBIAN PART OF THE DANUBE RIVER. Mirjana Lenhardt1, A. Hegediš2, B. Mićković2, Željka Višnjić Jeftić2, Marija Smederevac2, I. Jarić2, G. Cvijanović2, and Z. Gačić2. 1Siniša Stanković Institute for Biological Research, 11060 Belgrade, Serbia and 2Center for Multidisciplinary Studies, University of Belgrade, 11000 Belgrade, Serbia Key words: Non-native fish, Polyodon spathula, the Danube, Serbia UDC597.423(7:497.11) The North American paddlefish, Polyodon spathula, is one of Plovdiv and Vidin regions in Bulgaria (Hubenova et al., two living species of paddlefishes, the other being the Chinese 2005). paddlefish, Psephurus gladius. Although P. spathula was once abundant throughout the Mississippi River basin, since the be- In May 2006, a specimen of P. spathula was caught by ginning of the 20th century populations have declined dramati- professional fishermen near Prahovo in the Serbian part of the cally in most areas (Graham, 1997). Polyodon spathula is Danube River (river km 861). According to them, more speci- successfully reared in aquaculture, and, like sturgeon, is highly mens of different size and weight were caught at that time. The valued for its grayish-black roe (which is processed into caviar) specimens were caught with a floating drift net (100 x 4.5 m) and for its boneless firm white meat (Mims, 2001). with mesh size of 3.25 cm. They were identified according to Polyodon spathula is classified as vulnerable (VU) on the Page and Burr(1991). One specimen (Fig. 1) was deposi- IUCN Red List, and its international trade is restricted under ted in the collection of the Natural History Museum in Belgra- Appendix II of the CITES (since 11 June 1992). -

Leo Semenovich Berg and the Biology of Acipenseriformes: a Dedication
Environmental Biology of Fishes 48: 15–22, 1997. 1997 Kluwer Academic Publishers. Printed in the Netherlands. Leo Semenovich Berg and the biology of Acipenseriformes: a dedication Vadim J. Birstein1 & William E. Bemis2 1 The Sturgeon Society, 331 West 57th Street, Suite 159, New York, NY 10019, U.S.A. 2 Department of Biology and Graduate Program in Organismic and Evolutionary Biology, University of Massachusetts, Amherst, MA 01003, U.S.A. Received 5.3.1996 Accepted 23.5.1996 Key words: T. Dobzhansky, A. Sewertzoff, T. Lysenko, Paleonisciformes, biogeography This volume is dedicated to the memory of Leo Semenovich Berg (1876–1950), a Russian ichthyologist and geographer. In the foreword to the English translation of Berg’s remarkable treatise, ‘Nomogenesis or evolu- tion according to law’, Theodosius Dobzhansky wrote: ‘Berg was one of the outstanding intellects among Russian scientists. The breadth of his interests and the depth as well as the amplitude of his scholarship were remarkable. He had the reputation of being a ‘walking library’, because of the amount of information he could produce from his memory’ (Dobzhansky 1969, p. xi). Berg was prolific, publishing 217 papers and monographs on ichthyology, 30 papers on general zoology and biology, 20 papers on paleontology, 32 papers on zoogeo- graphy, 320 papers and monographs on geography, geology, and ethnography, as well as 290 biographies, obituaries, and popular articles (Berg 1955, Sokolov 1955). Berg was born 120 years ago, on 14 March 1876, in Sciences. Berg was never formally recognized by the town of Bendery. According to laws of the Rus- the Soviet Academy for his accomplishments in sian Empire, Berg could not enter the university as biology, and only later (1946) was he elected a mem- a Jew, so he was baptized and became a Lutheran, ber of the Geography Branch of the Soviet Acade- which allowed him to study and receive his diploma my of Sciences (Figure 1). -
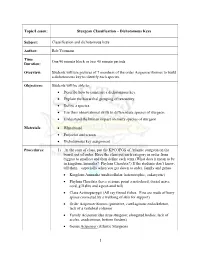
Sturgeon Classification – Dichotomous Keys Subject
Topic/Lesson: Sturgeon Classification – Dichotomous Keys Subject: Classification and dichotomous keys Author: Rob Yeomans Time One 90 minute block or two 45 minute periods Duration: Overview: Students will use pictures of 7 members of the order Acipenseriformes to build a dichotomous key to identify each species. Objectives: Students will be able to: • Describe how to construct a dichotomous key. • Explain the hierarchal grouping of taxonomy. • Define a species. • Use their observational skills to differentiate species of sturgeon. • Understand the human impact on many species of sturgeon. Materials: • Whiteboard • Projector and screen • Dichotomous key assignment Procedures: 1) At the start of class, put the KPCOFGS of Atlantic sturgeon on the board, out of order. Have the class put each category in order from biggest to smallest and then define each term (What does it mean to be in kingdom Animalia? Phylum Chordata?) If the students don’t know, tell them—especially when you get down to order, family and genus. • Kingdom Animalia (multicellular, heterotrophic, eukaryotic) • Phylum Chordata (have at some point a notochord, dorsal nerve cord, gill slits and a post-anal tail) • Class Actinopterygii (All ray finned fishes. Fins are made of bony spines connected by a webbing of skin for support) • Order Acipenseriformes (primitive, cartilaginous endoskeleton, lack of a vertebral column) • Family Acipenseridae (true sturgeon; elongated bodies, lack of scales, anadromous, bottom feeders) • Genus Acipenser (Atlantic Sturgeon) 1 • Species oxyrinchus Comment to the class that there are actually two subspecies of oxyrinchus. Acipenser oxyrinchus oxyrinchus is the Atlantic sturgeon and Acipenser oxyrinchus desotoi is the Gulf sturgeon 2) Reinforce the fact that sturgeon are a primitive fish and fossils have been found dating back 144-65 million years ago. -

Brenda Rivera and Antonio R. Rivera V
Brigham Young University Law School BYU Law Digital Commons Utah Court of Appeals Briefs 1993 Brenda Rivera and Antonio R. Rivera v. Clayton S. Wilde M.D. : Brief of Appellee Utah Court of Appeals Follow this and additional works at: https://digitalcommons.law.byu.edu/byu_ca1 Part of the Law Commons Original Brief Submitted to the Utah Court of Appeals; digitized by the Howard W. Hunter Law Library, J. Reuben Clark Law School, Brigham Young University, Provo, Utah; machine-generated OCR, may contain errors. Elliott .J Williams; Bruce H. Jensen; Williams and Hunt; Attorneys for Defendant Robert B. Sykes; Tamara J. Hauge; Sykes and Vilos, P.C.; Attorneys for Plaintiff Recommended Citation Brief of Appellee, Rivera v. Wilde, No. 930366 (Utah Court of Appeals, 1993). https://digitalcommons.law.byu.edu/byu_ca1/5284 This Brief of Appellee is brought to you for free and open access by BYU Law Digital Commons. It has been accepted for inclusion in Utah Court of Appeals Briefs by an authorized administrator of BYU Law Digital Commons. Policies regarding these Utah briefs are available at http://digitalcommons.law.byu.edu/utah_court_briefs/policies.html. Please contact the Repository Manager at [email protected] with questions or feedback. °l30^frk-6& IN THE UTAH COURT OF APPEALS BRENDA RIVERA, COURT OP APPEALS Plaintiff/Appellant, Appeal No. 930366-CA v. (Consolidated Cases) CLAYTON S. WILDE, M.D., Defendant/Appellee Priority No. 15 ANTONIO R. RIVERA, by and through his Guardian Ad Litem Tony Rivera, also known as Antonio R. Rivera, Plaintiff/Appellant, v. CLAYTON S. WILDE, M.D., Defendant/Appellee. -

The Key Threats to Sturgeons and Measures for Their Protection in the Lower Danube Region
THE KEY THREATS TO STURGEONS AND MEASURES FOR THEIR PROTECTION IN THE LOWER DANUBE REGION MIRJANA LENHARDT* Institute for Biological Research, Serbia IVAN JARIĆ, GORČIN CVIJANOVIĆ AND MARIJA SMEDEREVAC-LALIĆ Center for Multidisciplinary Studies, Serbia Abstract The six native sturgeon species have been commercially harvested in the Danube Basin for more than 2,000 years, with rapid decrease in catch by mid 19th century. Addi- tional negative effect on sturgeon populations in the Danube River was river regulation in Djerdap region, due to navigation in the late 19th century, as well as dam construction in the second half of 20th century that blocked sturgeon spawning migrations. Beside over- fishing and habitat loss, illegal trade, life history characteristics of sturgeon, lack of effective management (due to lack of transboundary cooperation and change in political situa- tion in Lower Danube Region countries) and pollution all pose serious threats on sturgeon populations in Lower Danube Region. International measures established by the Conven- tion on International Trade in Endangered Species (CITES) in late 20th century, listing of beluga (Huso huso) as an endangered species under the U.S. Endangered Species Act, as well as development of Action plan for conservation of sturgeons in the Danube River Basin, had significant impact on activities related to sturgeon protection at beginning of 21st century. These actions were aimed towards diminishment of pressure on natural sturgeon populations and aquaculture development in countries of Lower Danube Region. The main goal of the Action Plan was to raise public awareness and to create a common framework for implementation of urgent measures. -

2012 Wildearth Guardians and Friends of Animals Petition to List
PETITION TO LIST Fifteen Species of Sturgeon UNDER THE U.S. ENDANGERED SPECIES ACT Submitted to the U.S. Secretary of Commerce, Acting through the National Oceanic and Atmospheric Administration and the National Marine Fisheries Service March 8, 2012 Petitioners WildEarth Guardians Friends of Animals 1536 Wynkoop Street, Suite 301 777 Post Road, Suite 205 Denver, Colorado 80202 Darien, Connecticut 06820 303.573.4898 203.656.1522 INTRODUCTION WildEarth Guardians and Friends of Animals hereby petitions the Secretary of Commerce, acting through the National Marine Fisheries Service (NMFS)1 and the National Oceanic and Atmospheric Administration (NOAA) (hereinafter referred as the Secretary), to list fifteen critically endangered sturgeon species as “threatened” or “endangered” under the Endangered Species Act (ESA) (16 U.S.C. § 1531 et seq.). The fifteen petitioned sturgeon species, grouped by geographic region, are: I. Western Europe (1) Acipenser naccarii (Adriatic Sturgeon) (2) Acipenser sturio (Atlantic Sturgeon/Baltic Sturgeon/Common Sturgeon) II. Caspian Sea/Black Sea/Sea of Azov (3) Acipenser gueldenstaedtii (Russian Sturgeon) (4) Acipenser nudiventris (Ship Sturgeon/Bastard Sturgeon/Fringebarbel Sturgeon/Spiny Sturgeon/Thorn Sturgeon) (5) Acipenser persicus (Persian Sturgeon) (6) Acipenser stellatus (Stellate Sturgeon/Star Sturgeon) III. Aral Sea and Tributaries (endemics) (7) Pseudoscaphirhynchus fedtschenkoi (Syr-darya Shovelnose Sturgeon/Syr Darya Sturgeon) (8) Pseudoscaphirhynchus hermanni (Dwarf Sturgeon/Little Amu-Darya Shovelnose/Little Shovelnose Sturgeon/Small Amu-dar Shovelnose Sturgeon) (9) Pseudoscaphirhynchus kaufmanni (False Shovelnose Sturgeon/Amu Darya Shovelnose Sturgeon/Amu Darya Sturgeon/Big Amu Darya Shovelnose/Large Amu-dar Shovelnose Sturgeon/Shovelfish) IV. Amur River Basin/Sea of Japan/Sea of Okhotsk (10) Acipenser mikadoi (Sakhalin Sturgeon) (11) Acipenser schrenckii (Amur Sturgeon) (12) Huso dauricus (Kaluga) V.